Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response, and lateral root growth
- PMID: 11449059
- PMCID: PMC139551
- DOI: 10.1105/tpc.010158
Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response, and lateral root growth
Abstract
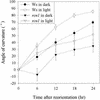
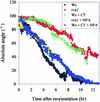
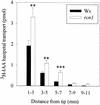
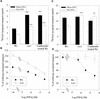
Auxin transport is required for important growth and developmental processes in plants, including gravity response and lateral root growth. Several lines of evidence suggest that reversible protein phosphorylation regulates auxin transport. Arabidopsis rcn1 mutant seedlings exhibit reduced protein phosphatase 2A activity and defects in differential cell elongation. Here we report that reduced phosphatase activity alters auxin transport and dependent physiological processes in the seedling root. Root basipetal transport was increased in rcn1 or phosphatase inhibitor-treated seedlings but showed normal sensitivity to the auxin transport inhibitor naphthylphthalamic acid (NPA). Phosphatase inhibition reduced root gravity response and delayed the establishment of differential auxin-induced gene expression across a gravity-stimulated root tip. An NPA treatment that reduced basipetal transport in rcn1 and cantharidin-treated wild-type plants also restored a normal gravity response and asymmetric auxin-induced gene expression, indicating that increased basipetal auxin transport impedes gravitropism. Increased auxin transport in rcn1 or phosphatase inhibitor-treated seedlings did not require the AGR1/EIR1/PIN2/WAV6 or AUX1 gene products. In contrast to basipetal transport, root acropetal transport was normal in phosphatase-inhibited seedlings in the absence of NPA, although it showed reduced NPA sensitivity. Lateral root growth also exhibited reduced NPA sensitivity in rcn1 seedlings, consistent with acropetal transport controlling lateral root growth. These results support the role of protein phosphorylation in regulating auxin transport and suggest that the acropetal and basipetal auxin transport streams are differentially regulated.
Figures
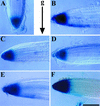
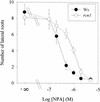
References
-
- Bennett, M.J., Marchant, A., Green, H.G., May, S.T., Ward, S.P., Millner, P.A., Walker, A.R., Schulz, B., and Feldmann, K.A. (1996). Arabidopsis AUX1 gene: A permease-like regulator of root gravitropism. Science 273, 948–950. - PubMed
-
- Berleth, T., and Jurgens, G. (1993). The role of the monopteros gene in organising the basal body region of the Arabidopsis embryo. Development 118, 575–587.
-
- Bernasconi, P. (1996). Effect of synthetic and natural protein tyrosine kinase inhibitors on auxin efflux in zucchini (Cucurbita pepo) hypocotyls. Physiol. Plant. 96, 205–210.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

