Sodium lauryl sulfate abrogates human immunodeficiency virus infectivity by affecting viral attachment
- PMID: 11451679
- PMCID: PMC90636
- DOI: 10.1128/AAC.45.8.2229-2237.2001
Sodium lauryl sulfate abrogates human immunodeficiency virus infectivity by affecting viral attachment
Abstract
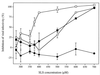
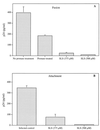
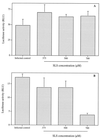
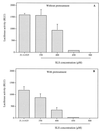
The microbicidal activity of sodium lauryl sulfate (SLS) against human immunodeficiency virus type 1 (HIV-1) was studied in cultured cells. Pretreatment of HIV-1(NL4-3) with SLS decreased, in a concentration-dependent manner, its infectivity when using 1G5 as target cells. In the absence of a viral pretreatment period or when 1G5 cells were pretreated with SLS, the surfactant-induced inactivation of viral infectivity was less pronounced, especially at concentrations between 375 and 550 microM. SLS had no effect on HIV-1 when the virus was adsorbed to 1G5 cells by a 2-h incubation period. SLS almost completely inhibited the fusion process by decreasing the attachment of HIV-1 to target cells. SLS also inhibited the infectivity of HIV-1-based luciferase reporter viruses pseudotyped with the amphotropic murine leukemia virus envelope (which enters cells in a CD4-, CCR5-, and CXCR4-independent manner), indicating that SLS may inactivate other envelope viruses. In contrast, no effect was seen with vesicular stomatitis virus envelope glycoprotein G (which enters cells through receptor-mediated endocytosis) pretreated with up to 700 microM SLS. SLS also decreased, in a dose-dependent manner, the HIV-1-dependent syncytium formation between 1G5 and J1.1 cells after a 24-h incubation. The reduction of luciferase activity was more pronounced when J1.1 cells (which express HIV-1 proteins on their surface) were pretreated with SLS rather than 1G5 cells. Taken together, our results suggest that SLS could represent a candidate of choice for use in vaginal microbicides to prevent the sexual transmission of HIV and possibly other pathogens causing sexually transmitted diseases.
Figures
References
-
- Aguilar-Cordova E, Chinen J, Donehower L, Lewis D E, Belmont J W. A sensitive reporter cell line for HIV-1 tat activity, HIV-1 inhibitors, and T cell activation effects. AIDS Res Hum Retrovir. 1994;10:295–301. - PubMed
-
- Alexander N J. Future contraceptives. Sci Am. 1995;273:136–141. - PubMed
-
- Bourinbaiar A S, Lee-Huang S. Comparative in vitro study of contraceptive agents with anti-HIV activity: gramicidin, nonoxynol-9 and gossypol. Contraception. 1994;49:131–137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

