Natural antibiotic susceptibilities of Edwardsiella tarda, E. ictaluri, and E. hoshinae
- PMID: 11451681
- PMCID: PMC90638
- DOI: 10.1128/AAC.45.8.2245-2255.2001
Natural antibiotic susceptibilities of Edwardsiella tarda, E. ictaluri, and E. hoshinae
Abstract
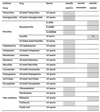
The natural antibiotic susceptibilities to 71 antibiotics of 102 Edwardsiella strains belonging to E. tarda (n = 42), E. ictaluri (n = 41), and E. hoshinae (n = 19) were investigated. MICs were determined using a microdilution procedure according to NCCLS criteria and German standards. All edwardsiellae were naturally sensitive to tetracyclines, aminoglycosides, most beta-lactams, quinolones, antifolates, chloramphenicol, nitrofurantoin, and fosfomycin. Edwardsiella species were naturally resistant to macrolides, lincosamides, streptogramins, glycopeptides, rifampin, fusidic acid, and oxacillin. Although slight species-dependent differences in natural susceptibilities to some antibiotics (e.g., macrolides and cefaclor) were seen, differences in natural susceptibility affecting clinical assessment criteria were only seen with benzylpenicillin. Whereas E. tarda was naturally resistant to benzylpenicillin, E. hoshinae was naturally sensitive. Natural sensitivity and resistance to this penicillin were found among the strains of E. ictaluri. The observed oxacillin sensitivity of E. ictaluri was attributed to the failure of the species to grow at higher salt concentrations found in oxacillin-containing microtiter plates. The present study describes a database concerning the natural susceptibility of Edwardsiella species to a wide range of antibiotics, which can be applied to validate forthcoming antibiotic susceptibility tests of these microorganisms.
Figures





References
-
- Bengoechea J A, Brandenburg K, Seydel U, Diaz R, Moriyon I. Yersinia pseudotuberculosis and Yersinia pestis show increased outer membrane permeability to hydrophobic agents which correlates with lipopolysaccharide acyl-chain fluidity. Microbiology. 1998;144:1517–1526. - PubMed
-
- Bergan T, Lolekha S, Cheong M K, Poh C L, Doencham S, Charoenpipop D. Effect of recent antibacterial agents against bacteria causing diarrhoea. Scand J Infect Dis. 1988;56:7–10. - PubMed
-
- Clark R B, Lister P D, Janda J M. In vitro susceptibilities of Edwardsiella tarda to 22 antibiotics and antibiotic-β-lactamase-inhibitor agents. Diagn Microbiol Infect Dis. 1991;14:173–175. - PubMed
-
- Comité de l'Antibiogramme de la Société Française de Microbiologie. Statement. Pathol Biol. 1998;46:I–XVI.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

