GT160-246, a toxin binding polymer for treatment of Clostridium difficile colitis
- PMID: 11451694
- PMCID: PMC90651
- DOI: 10.1128/AAC.45.8.2340-2347.2001
GT160-246, a toxin binding polymer for treatment of Clostridium difficile colitis
Abstract
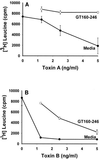
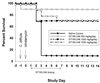
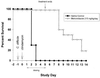
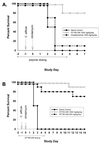
GT160-246, a high-molecular-weight soluble anionic polymer, was tested in vitro and in vivo for neutralization of Clostridium difficile toxin A and B activities. Five milligrams of GT160-246 per ml neutralized toxin-mediated inhibition of protein synthesis in Vero cells induced by 5 ng of toxin A per ml or 1.25 ng of toxin B per ml. In ligated rat ileal loops, 1 mg of GT160-246 neutralized fluid accumulation caused by 5 microg of toxin A. At doses as high as 80 mg/loop, cholestyramine provided incomplete neutralization of fluid accumulation caused by 5 microg of toxin A. GT160-246 protected 80% of the hamsters from mortality caused by infection with C. difficile, whereas cholestyramine protected only 10% of animals. Treatment of C. difficile-infected hamsters with metronidazole initially protected 100% of the hamsters from mortality, but upon removal of treatment, 80% of the hamsters had relapses and died. In contrast, removal of GT160-246 treatment did not result in disease relapse in the hamsters. GT160-246 showed no antimicrobial activity in tests with a panel of 16 aerobic bacteria and yeast and 22 anaerobic bacteria and did not interfere with the in vitro activities of most antibiotics. GT160-246 offers a novel, nonantimicrobial treatment of C. difficile disease in humans.
Figures

Similar articles
-
Vaccination with parenteral toxoid B protects hamsters against lethal challenge with toxin A-negative, toxin B-positive clostridium difficile but does not prevent colonization.J Infect Dis. 2012 Jan 1;205(1):128-33. doi: 10.1093/infdis/jir688. Epub 2011 Nov 28. J Infect Dis. 2012. PMID: 22124129
-
Toxin-binding treatment for Clostridium difficile: a review including reports of studies with tolevamer.Int J Antimicrob Agents. 2009 Jan;33(1):4-7. doi: 10.1016/j.ijantimicag.2008.07.011. Epub 2008 Sep 18. Int J Antimicrob Agents. 2009. PMID: 18804351 Review.
-
Binary toxin-producing, large clostridial toxin-negative Clostridium difficile strains are enterotoxic but do not cause disease in hamsters.J Infect Dis. 2006 Apr 15;193(8):1143-50. doi: 10.1086/501368. Epub 2006 Mar 6. J Infect Dis. 2006. PMID: 16544255
-
Review article: tolevamer, a novel toxin-binding polymer: overview of preclinical pharmacology and physicochemical properties.Aliment Pharmacol Ther. 2006 Dec;24(11-12):1525-34. doi: 10.1111/j.1365-2036.2006.03157.x. Aliment Pharmacol Ther. 2006. PMID: 17206941 Review.
-
Co-infection of hamsters with toxin A or toxin B-deficient Clostridium difficile strains.Pol J Microbiol. 2005;54(4):301-4. Pol J Microbiol. 2005. PMID: 16599301
Cited by
-
Clostridium difficile toxins: mechanism of action and role in disease.Clin Microbiol Rev. 2005 Apr;18(2):247-63. doi: 10.1128/CMR.18.2.247-263.2005. Clin Microbiol Rev. 2005. PMID: 15831824 Free PMC article. Review.
-
Orally administered P22 phage tailspike protein reduces salmonella colonization in chickens: prospects of a novel therapy against bacterial infections.PLoS One. 2010 Nov 22;5(11):e13904. doi: 10.1371/journal.pone.0013904. PLoS One. 2010. PMID: 21124920 Free PMC article.
-
The roles of host and pathogen factors and the innate immune response in the pathogenesis of Clostridium difficile infection.Mol Immunol. 2015 Feb;63(2):193-202. doi: 10.1016/j.molimm.2014.09.005. Epub 2014 Sep 18. Mol Immunol. 2015. PMID: 25242213 Free PMC article. Review.
-
Clostridium difficile infection in hospitals: risk factors and responses.CMAJ. 2004 Jul 6;171(1):45-6. doi: 10.1503/cmaj.1040966. CMAJ. 2004. PMID: 15238495 Free PMC article. No abstract available.
-
Tolevamer is not efficacious in the neutralization of cytotoxin in a human gut model of Clostridium difficile infection.Antimicrob Agents Chemother. 2009 May;53(5):2202-4. doi: 10.1128/AAC.01085-08. Epub 2009 Feb 17. Antimicrob Agents Chemother. 2009. PMID: 19223641 Free PMC article.
References
-
- Anonymous. ASHP therapeutic position statement on the preferential use of metronidazole for the treatment of Clostridium difficile-associated disease. Am J Health Syst Pharm. 1998;55:1407–1411. - PubMed
-
- Burbige E J, Milligan F D. Pseudomembranous colitis. Association with antibiotics and therapy with cholestyramine. JAMA. 1975;231:1157–1158. - PubMed
-
- Castagliuolo I, LaMont J T, Qiu B, Nikulasson S T, Pothoulakis C. A receptor decoy inhibits the enterotoxic effects of Clostridium difficile toxin A in rat ileum. Gastroenterology. 1996;111:433–438. - PubMed
-
- Fiorentini C, Thelestam M. Clostridium difficile toxin A and its effects on cells. Toxicon. 1991;29:543–567. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

