Topological and mutational analysis of Saccharomyces cerevisiae Ste14p, founding member of the isoprenylcysteine carboxyl methyltransferase family
- PMID: 11451995
- PMCID: PMC55642
- DOI: 10.1091/mbc.12.7.1957
Topological and mutational analysis of Saccharomyces cerevisiae Ste14p, founding member of the isoprenylcysteine carboxyl methyltransferase family
Abstract
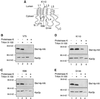
Eukaryotic proteins that terminate in a CaaX motif undergo three processing events: isoprenylation, C-terminal proteolytic cleavage, and carboxyl methylation. In Saccharomyces cerevisiae, the latter step is mediated by Ste14p, an integral endoplasmic reticulum membrane protein. Ste14p is the founding member of the isoprenylcysteine carboxyl methyltransferase (ICMT) family, whose members share significant sequence homology. Because the physiological substrates of Ste14p, such as Ras and the yeast a-factor precursor, are isoprenylated and reside on the cytosolic side of membranes, the Ste14p residues involved in enzymatic activity are predicted to be cytosolically disposed. In this study, we have investigated the topology of Ste14p by analyzing the protease protection of epitope-tagged versions of Ste14p and the glycosylation status of Ste14p-Suc2p fusions. Our data lead to a topology model in which Ste14p contains six membrane spans, two of which form a helical hairpin. According to this model most of the Ste14p hydrophilic regions are located in the cytosol. We have also generated ste14 mutants by random and site-directed mutagenesis to identify residues of Ste14p that are important for activity. Notably, four of the five loss-of-function mutations arising from random mutagenesis alter residues that are highly conserved among the ICMT family. Finally, we have identified a novel tripartite consensus motif in the C-terminal region of Ste14p. This region is similar among all ICMT family members, two phospholipid methyltransferases, several ergosterol biosynthetic enzymes, and a group of bacterial open reading frames of unknown function. Site-directed and random mutations demonstrate that residues in this region play a critical role in the function of Ste14p.
Figures








References
-
- Abeliovich H, Grote E, Novick P, Ferro-Novick S. Tlg2p, a yeast syntaxin homolog that resides on the Golgi and endocytic structures. J Biol Chem. 1998;273:11719–11727. - PubMed
-
- Ashby MN, Errada PR, Boyartchuk VL, Rine J. Isolation and DNA sequence of the STE14gene encoding farnesyl cysteine: carboxyl methyltransferase. Yeast. 1993;9:907–913. - PubMed
-
- Bergo MO, Leung GK, Ambroziak P, Otto JC, Casey PJ, Young SG. Targeted inactivation of the isoprenylcysteine carboxyl methyltransferase gene causes mislocalization of K-Ras in mammalian cells. J Biol Chem. 2000;275:17605–17610. - PubMed
-
- Berkower C. Analysis of STE6, a Saccharomyces cerevisiae ATP Binding Cassette (ABC) Protein. Ph.D. Thesis. Baltimore, MD: Johns Hopkins University; 1995.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

