The Golgi complex is a microtubule-organizing organelle
- PMID: 11452002
- PMCID: PMC55652
- DOI: 10.1091/mbc.12.7.2047
The Golgi complex is a microtubule-organizing organelle
Abstract
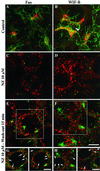
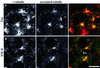
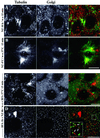
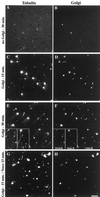
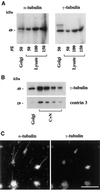
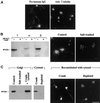
We show that the Golgi complex can directly stimulate microtubule nucleation in vivo and in vitro and thus behaves as a potent microtubule-organizing organelle in interphase cells. With the use of nocodazole wash-out experiments in hepatic cells, we found that the occurrence of noncentrosomal, early stabilized microtubules is highly correlated with the subcellular localization of Golgi membranes. With the use of in vitro reconstituted microtubule assembly systems with or without cytosol, we also found that, in contrast to centrosomally attached microtubules, the distal ends of Golgi-attached microtubules are remotely stabilized in a way that requires additional cytosolic component(s). Finally, we demonstrate that Golgi-based microtubule nucleation is direct and involves a subset of gamma-tubulin bound to the cytoplasmic face of the organelle.
Figures


References
-
- Biou D, Monnet D, Miller D, Feger J, Durand G. An immunochemical procedure to evaluate the degree of desialylation of α1-acid glycoprotein in rat serum. J Immunol Methods. 1984;74:267–271. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

