Activation of APE/Ref-1 redox activity is mediated by reactive oxygen species and PKC phosphorylation
- PMID: 11452037
- PMCID: PMC55809
- DOI: 10.1093/nar/29.14.3116
Activation of APE/Ref-1 redox activity is mediated by reactive oxygen species and PKC phosphorylation
Abstract
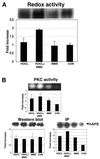
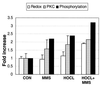
Reactive oxygen species (ROS) arise through normal cellular aerobic respiration, and, in combination with external sources such as ionizing radiation, cigarette tar and smoke, and particulate matter generated by combustion, can have a profound negative effect on cellular macromolecules such as DNA that may lead to a number of human pathological disorders including accelerated aging and cancer. A major end product of ROS damage to DNA is the formation of apurinic/apyrimidinic (AP) sites, which without removal are known to halt mRNA and DNA synthesis, or act as non-coding lesions resulting in the increased generation of DNA mutations. In human cells, the major enzyme in correcting the deleterious effects of AP sites in DNA is through the participation of AP endonuclease (APE), which initiates the removal of baseless sites in DNA through the catalytic scission of the phosphodiester bond 5' and adjacent to an AP site. Interestingly, APE also possesses an activity (Ref-1) that controls the redox status of a number of transcription factors including Fos and Jun. The means by which APE/Ref-1 is directed to carry out such disparate roles are unknown. The presence of a number of phosphorylation sites scattered throughout both functional domains of APE/Ref-1 however offered one possible mechanism that we reasoned could play a role in dictating how this protein responds to different stimuli. Here we show that the in vitro redox activity of APE/Ref-1 is stimulated by PKC phosphorylation. Furthermore, when human cells were exposed to the PKC activator phorbol 12-myristate 13-acetate, an increase in redox activity was observed that corresponded to an increase in the phosphorylation status of APE/Ref-1. Importantly, human cells exposed to the oxidizing agent hypochlorite, followed by methyl methanesulfanate, responded with an increase in redox activity by APE/Ref-1 that also involved an increase in PKC activity and a corresponding increase in the phosphorylation of APE/Ref-1. These results suggest that the ability of APE/Ref-1 to perform its in vivo redox function is correlated to its susceptibility to PKC phosphorylation that notably occurs in response to DNA damaging agents.
Figures



Similar articles
-
Phosphorylation of the DNA repair protein APE/REF-1 by CKII affects redox regulation of AP-1.Oncogene. 1999 Jan 28;18(4):1033-40. doi: 10.1038/sj.onc.1202394. Oncogene. 1999. PMID: 10023679
-
The DNA repair activity of human redox/repair protein APE/Ref-1 is inactivated by phosphorylation.Cancer Res. 1997 Dec 15;57(24):5457-9. Cancer Res. 1997. PMID: 9407949
-
Helicobacter pylori and H2O2 increase AP endonuclease-1/redox factor-1 expression in human gastric epithelial cells.Gastroenterology. 2004 Sep;127(3):845-58. doi: 10.1053/j.gastro.2004.06.017. Gastroenterology. 2004. PMID: 15362040
-
Human APE/Ref-1 protein.Int J Biochem Cell Biol. 2000 Sep;32(9):925-9. doi: 10.1016/s1357-2725(00)00045-5. Int J Biochem Cell Biol. 2000. PMID: 11084372 Review.
-
Anticancer clinical utility of the apurinic/apyrimidinic endonuclease/redox factor-1 (APE/Ref-1).Chin J Cancer. 2010 Mar;29(3):333-9. doi: 10.5732/cjc.009.10285. Chin J Cancer. 2010. PMID: 20193121 Review.
Cited by
-
Effects of gemcitabine on APE/ref-1 endonuclease activity in pancreatic cancer cells, and the therapeutic potential of antisense oligonucleotides.Br J Cancer. 2004 Sep 13;91(6):1166-73. doi: 10.1038/sj.bjc.6602080. Br J Cancer. 2004. PMID: 15316562 Free PMC article.
-
Ape1/Ref-1 induces glial cell-derived neurotropic factor (GDNF) responsiveness by upregulating GDNF receptor alpha1 expression.Mol Cell Biol. 2009 Apr;29(8):2264-77. doi: 10.1128/MCB.01484-08. Epub 2009 Feb 2. Mol Cell Biol. 2009. PMID: 19188437 Free PMC article.
-
Human apurinic/apyrimidinic endonuclease 1.Antioxid Redox Signal. 2014 Feb 1;20(4):678-707. doi: 10.1089/ars.2013.5492. Epub 2013 Aug 20. Antioxid Redox Signal. 2014. PMID: 23834463 Free PMC article. Review.
-
Peroxiredoxin 1 interacts with and blocks the redox factor APE1 from activating interleukin-8 expression.Sci Rep. 2016 Jul 8;6:29389. doi: 10.1038/srep29389. Sci Rep. 2016. PMID: 27388124 Free PMC article.
-
ATF4-dependent oxidative induction of the DNA repair enzyme Ape1 counteracts arsenite cytotoxicity and suppresses arsenite-mediated mutagenesis.Mol Cell Biol. 2007 Dec;27(24):8834-47. doi: 10.1128/MCB.00974-07. Epub 2007 Oct 15. Mol Cell Biol. 2007. PMID: 17938202 Free PMC article.
References
-
- Lindahl T. and Andersson,A. (1972) Rate of chain breakage at apurinic sites in double-stranded deoxyribonucleic acid. Biochemistry, 11, 3618–3623. - PubMed
-
- Loeb L.A. and Preston,B.D. (1986) Mutagenesis by apurinic/apyrimidinic sites. Annu. Rev. Genet., 20, 201–230. - PubMed
-
- Kane C.M. and Linn,S. (1981) Purification and characterization of an apurinic/apyrimidinic endonuclease from HeLa cells. J. Biol. Chem., 256, 3405–3414. - PubMed
-
- Matsumoto Y. and Kim,K. (1995) Excision of deoxyribose phosphate residues by DNA polymerase beta during DNA repair. Science, 269, 699–702. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

