Accessibility of DNA polymerases to repair synthesis during nucleotide excision repair in yeast cell-free extracts
- PMID: 11452038
- PMCID: PMC55800
- DOI: 10.1093/nar/29.14.3123
Accessibility of DNA polymerases to repair synthesis during nucleotide excision repair in yeast cell-free extracts
Abstract
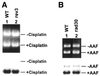
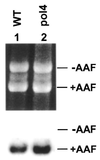
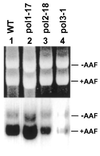
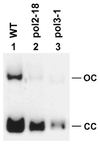
Nucleotide excision repair (NER) removes a variety of DNA lesions. Using a yeast cell-free repair system, we have analyzed the repair synthesis step of NER. NER was proficient in yeast mutant cell-free extracts lacking DNA polymerases (Pol) beta, zeta or eta. Base excision repair was also proficient without Polbeta. Repair synthesis of NER was not affected by thermal inactivation of the temperature-sensitive mutant Polalpha (pol1-17), but was reduced after thermal inactivation of the temperature-sensitive mutant Poldelta (pol3-1) or Polvarepsilon (pol2-18). Residual repair synthesis was observed in pol3-1 and pol2-18 mutant extracts, suggesting a repair deficiency rather than a complete repair defect. Deficient NER in pol3-1 and pol2-18 mutant extracts was specifically complemented by purified yeast Poldelta and Polvarepsilon, respectively. Deleting the polymerase catalytic domain of Polvarepsilon (pol2-16) also led to a deficient repair synthesis during NER, which was complemented by purified yeast Polvarepsilon, but not by purified yeast Poleta. These results suggest that efficient repair synthesis of yeast NER requires both Poldelta and Polvarepsilon in vitro, and that the low fidelity Poleta is not accessible to repair synthesis during NER.
Figures




Similar articles
-
Proofreading activity of DNA polymerase Pol2 mediates 3'-end processing during nonhomologous end joining in yeast.PLoS Genet. 2008 Apr 25;4(4):e1000060. doi: 10.1371/journal.pgen.1000060. PLoS Genet. 2008. PMID: 18437220 Free PMC article.
-
DNA repair synthesis during base excision repair in vitro is catalyzed by DNA polymerase epsilon and is influenced by DNA polymerases alpha and delta in Saccharomyces cerevisiae.Mol Cell Biol. 1993 Feb;13(2):1051-8. doi: 10.1128/mcb.13.2.1051-1058.1993. Mol Cell Biol. 1993. PMID: 8423775 Free PMC article.
-
DNA ligation during excision repair in yeast cell-free extracts is specifically catalyzed by the CDC9 gene product.Biochemistry. 1999 Mar 2;38(9):2628-35. doi: 10.1021/bi982592s. Biochemistry. 1999. PMID: 10052932
-
Transcription coupled nucleotide excision repair in the yeast Saccharomyces cerevisiae: The ambiguous role of Rad26.DNA Repair (Amst). 2015 Dec;36:43-48. doi: 10.1016/j.dnarep.2015.09.006. Epub 2015 Sep 10. DNA Repair (Amst). 2015. PMID: 26429063 Review.
-
Nucleotide excision repair I: from E. coli to yeast.Trends Genet. 1993 May;9(5):173-7. doi: 10.1016/0168-9525(93)90164-d. Trends Genet. 1993. PMID: 8337754 Review.
Cited by
-
The high fidelity and unique error signature of human DNA polymerase epsilon.Nucleic Acids Res. 2011 Mar;39(5):1763-73. doi: 10.1093/nar/gkq1034. Epub 2010 Oct 29. Nucleic Acids Res. 2011. PMID: 21036870 Free PMC article.
-
DNA interstrand crosslink repair during G1 involves nucleotide excision repair and DNA polymerase zeta.EMBO J. 2006 Mar 22;25(6):1285-94. doi: 10.1038/sj.emboj.7600993. Epub 2006 Feb 16. EMBO J. 2006. PMID: 16482220 Free PMC article.
-
Proofreading activity of DNA polymerase Pol2 mediates 3'-end processing during nonhomologous end joining in yeast.PLoS Genet. 2008 Apr 25;4(4):e1000060. doi: 10.1371/journal.pgen.1000060. PLoS Genet. 2008. PMID: 18437220 Free PMC article.
-
DNA polymerase epsilon: a polymerase of unusual size (and complexity).Prog Nucleic Acid Res Mol Biol. 2008;82:101-45. doi: 10.1016/S0079-6603(08)00004-4. Prog Nucleic Acid Res Mol Biol. 2008. PMID: 18929140 Free PMC article. Review. No abstract available.
-
Ubc4 and Not4 regulate steady-state levels of DNA polymerase-α to promote efficient and accurate DNA replication.Mol Biol Cell. 2010 Sep 15;21(18):3205-19. doi: 10.1091/mbc.E09-06-0452. Epub 2010 Jul 21. Mol Biol Cell. 2010. PMID: 20660159 Free PMC article.
References
-
- Cleaver J.E. and Kraemer,K.H. (1989) Xeroderma pigmentosum. In Scriver,C.R., Beaudet,A.L., Sly,W.S. and Valle,D. (eds), The Metabolic Basis of Inherited Disease, 6th Edn. McGraw–Hill Book Co., New York, NY, pp. 2949–2971.
-
- Hanawalt P.C. (1994) Transcription-coupled repair and human disease. Science, 266, 1957–1958. - PubMed
-
- de Laat W.L., Jaspers,N.G. and Hoeijmakers,J.H. (1999) Molecular mechanism of nucleotide excision repair. Genes Dev., 13, 768–785. - PubMed
-
- Friedberg E.C., Walker,G.C. and Siede,W. (1995) DNA Repair and Mutagenesis. American Society of Microbiology Press, Washington, DC.
-
- Bhatia P.K., Wang,Z. and Friedberg,E.C. (1996) DNA repair and transcription. Curr. Opin. Genet. Dev., 6, 146–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

