Pharmacokinetic-pharmacodynamic model for perindoprilat regional haemodynamic effects in healthy volunteers and in congestive heart failure patients
- PMID: 11453887
- PMCID: PMC2014509
- DOI: 10.1046/j.0306-5251.2001.01410.x
Pharmacokinetic-pharmacodynamic model for perindoprilat regional haemodynamic effects in healthy volunteers and in congestive heart failure patients
Abstract
Aims: We compared the relationships between the plasma concentrations (C) of perindoprilat, active metabolite of the angiotensin I-converting enzyme inhibitor (ACEI) perindopril, and the effects (E) induced on plasma converting enzyme activity (PCEA) and brachial vascular resistance (BVR) in healthy volunteers (HV) and in congestive heart failure (CHF) patients after single oral doses of perindopril.
Methods: Six HV received three doses of perindopril (4, 8, 16 mg) in a placebo-controlled, randomized, double-blind, crossover study whereas 10 CHF patients received one dose (4 mg) in an open study. Each variable was determined before and 6-12 times after drug intake. E (% variations from baseline) were individually related to C (ng ml(-1)) by the Hill model E=Emax x Cgamma/(CE50gamma + Cgamma). When data showed a hysteresis loop, an effect compartment was used.
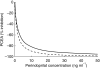
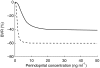
Results: (means+/-s.d.) In HV, relationships between C and E were direct whereas in CHF patients, they showed hysteresis loops with optimal k(e0) values of 0.13 +/- 0.16 and 0.13 +/- 0.07 h(-1) for PCEA and BVR, respectively. For PCEA, with Emax set to -100%, CE50 = 1.87 +/- 0.60 and 1.36 +/- 1.33 ng ml(-1) (P = 0.34) and gamma = 0.90 +/- 0.13 and 1.11 +/- 0.47 (P = 0.23) in HV and CHF patients, respectively. For BVR, Emax= -41 +/- 14% and -60 +/- 7% (P = 0.02), CE50 = 4.95 +/- 2.62 and 1.38 +/- 0.85 ng ml(-1) (P = 0.02), and gamma = 2.25 +/- 1.54 and 3.06 +/- 1.37 (P = 0.32) in HV and CHF patients, respectively.
Conclusions: Whereas concentration-effect relationships were similar in HV and CHF patients for PCEA blockade, they strongly differed for regional haemodynamics. This result probably expresses the different involvements, in HV and CHF patients, of angiotensinergic and nonangiotensinergic mechanisms in the haemodynamic effects of ACEIs.
Figures
Similar articles
-
Perindopril: in congestive heart failure.Drugs. 2002;62(9):1367-77; discussion 1378-9. doi: 10.2165/00003495-200262090-00013. Drugs. 2002. PMID: 12076191 Review.
-
Pharmacokinetic-pharmacodynamic model relating lisinopril plasma concentrations to regional hemodynamic effects in healthy volunteers.J Cardiovasc Pharmacol. 1996 Sep;28(3):470-8. doi: 10.1097/00005344-199609000-00018. J Cardiovasc Pharmacol. 1996. PMID: 8877596
-
Pharmacokinetic-pharmacodynamic model relating zabiciprilat plasma concentrations to brachial and femoral haemodynamic effects in normotensive volunteers.Br J Clin Pharmacol. 1998 Oct;46(4):383-93. doi: 10.1046/j.1365-2125.1998.00786.x. Br J Clin Pharmacol. 1998. PMID: 9803988 Free PMC article. Clinical Trial.
-
Pharmacokinetic-pharmacodynamic model for fantofarone cardiac and brachial haemodynamic effects in healthy volunteers.Br J Clin Pharmacol. 1999 Dec;48(6):801-10. doi: 10.1046/j.1365-2125.1999.00091.x. Br J Clin Pharmacol. 1999. PMID: 10594483 Free PMC article. Clinical Trial.
-
Distinctive properties of perindopril among converting enzyme inhibitors in congestive heart failure.Can J Cardiol. 1994 Nov;10 Suppl D:13D-16D. Can J Cardiol. 1994. PMID: 7954033 Review.
Cited by
-
Understanding the hysteresis loop conundrum in pharmacokinetic/pharmacodynamic relationships.J Pharm Pharm Sci. 2014;17(1):34-91. J Pharm Pharm Sci. 2014. PMID: 24735761 Free PMC article.
-
Population pharmacokinetic/pharmacodynamic analysis of AK111, an IL-17A monoclonal antibody, in subjects with moderate-to-severe plaque psoriasis.Front Pharmacol. 2022 Aug 16;13:966176. doi: 10.3389/fphar.2022.966176. eCollection 2022. Front Pharmacol. 2022. PMID: 36052126 Free PMC article.
-
Perindopril: in congestive heart failure.Drugs. 2002;62(9):1367-77; discussion 1378-9. doi: 10.2165/00003495-200262090-00013. Drugs. 2002. PMID: 12076191 Review.
-
Simultaneous determination of indapamide, perindopril and perindoprilat in human plasma or whole blood by UPLC-MS/MS and its pharmacokinetic application.J Pharm Anal. 2018 Oct;8(5):333-340. doi: 10.1016/j.jpha.2018.05.004. Epub 2018 May 19. J Pharm Anal. 2018. PMID: 30345148 Free PMC article.
-
The influence of heart failure on the pharmacokinetics of cardiovascular and non-cardiovascular drugs: a critical appraisal of the evidence.Br J Clin Pharmacol. 2019 Jan;85(1):20-36. doi: 10.1111/bcp.13760. Epub 2018 Oct 14. Br J Clin Pharmacol. 2019. PMID: 30194701 Free PMC article. Review.
References
-
- Kroboth PD, Schmith VD, Smith RB. Pharmacodynamic modelling. Application to new drug development. Clin Pharmacokinet. 1991;20:91–98. - PubMed
-
- Peck CC, Barr WH, Benet LZ, et al. Opportunities for integration of pharmacokinetics, pharmacodynamics, and toxicokinetics in rational drug development. Clin Pharmacol Ther. 1992;51:465–473. - PubMed
-
- Elliott HL, Donnelly R, Meredith PA, Reid JL. Predictability of antihypertensive responsiveness and α-adrenoceptor antagonism during prazosin treatment. Clin Pharmacol Ther. 1989;46:576–583. - PubMed
-
- Donnelly R, Meredith PA, Elliott HL, Reid JL. Kinetic-dynamic relations and individual responses to enalapril. Hypertension. 1990;15:301–309. - PubMed
-
- Donnelly R, Meredith PA, Miller SHK, Howie CA, Elliott HL. Pharmacodynamic modeling of the antihypertensive response to amlodipine. Clin Pharmacol Ther. 1993;54:303–310. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

