Dihydroergotamine: discrepancy between arterial, arteriolar and pharmacokinetic data
- PMID: 11453889
- PMCID: PMC2014503
- DOI: 10.1046/j.0306-5251.2001.01415.x
Dihydroergotamine: discrepancy between arterial, arteriolar and pharmacokinetic data
Abstract
Aims: To investigate the peripheral vascular effects and pharmacokinetics of dihydroergotamine (DHE) 0.5 mg after a single subcutaneous administration in humans.
Methods: A double-blind, placebo-controlled cross-over study was performed in 10 healthy male subjects. A wash-out period of 2 weeks separated the two study periods. During each period, just before and at regular intervals after drug administration, vascular measurements were performed and venous blood samples were drawn. Vessel wall properties were assessed at the brachial artery, by ultrasound and applanation tonometry. Blood pressure and heart rate were recorded with an oscillometric device. Forearm blood flow was measured with venous occlusion plethysmography. For all parameter-time curves the area under the curve (AUC) was calculated. Differences in AUC after placebo and DHE (DeltaAUC) were analysed and the time-course of the difference assessed. DHE pharmacokinetics were analysed according to a two-compartment open model with an absorption phase.
Results: AUC for blood pressure, heart rate and forearm vascular resistance did not change after DHE. Brachial artery diameter and compliance decreased (P < 0.01); DeltaAUC (95% confidence interval) equalled -8.81 mm h (-12.97/-4.65) and -0.98 mm2 kPa(-1) h (-1.61/-0.34), respectively. Diameter decreased (P < 0.05) from 1 until 24 h after DHE (peak decrease 9.7% at 10 h); compliance from 2 until 32 h (24.8% at 2 h). Time to reach maximum plasma concentration of DHE averaged 0.33 +/- 0.08 h (+/- s.e.mean); terminal half-life was 5.63 +/- 1.15 h.
Conclusions: DHE decreased diameter and compliance of the brachial artery whereas forearm vascular resistance remained unchanged. Thus, DHE acts on conduit arteries without affecting resistance arteries. Furthermore, a discrepancy was demonstrated between the plasma concentrations of DHE which rapidly reach peak levels and quickly decline, and its long lasting vasoconstrictor activity.
Figures
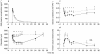
Similar articles
-
Relationship of dihydroergotamine pharmacokinetics, clinical efficacy, and nausea-A narrative review.Headache. 2025 Mar;65(3):527-535. doi: 10.1111/head.14877. Epub 2024 Nov 27. Headache. 2025. PMID: 39601088 Free PMC article. Review.
-
Intrapulmonary and intravenous administrations of dihydroergotamine mesylate have similar cardiovascular effects in the conscious dog.Br J Pharmacol. 2008 Jul;154(6):1254-65. doi: 10.1038/bjp.2008.187. Epub 2008 May 26. Br J Pharmacol. 2008. PMID: 18500365 Free PMC article.
-
A Phase 1, Randomized, Open-Label, Safety, Tolerability, and Comparative Bioavailability Study of Intranasal Dihydroergotamine Powder (STS101), Intramuscular Dihydroergotamine Mesylate, and Intranasal DHE Mesylate Spray in Healthy Adult Subjects.Headache. 2020 Apr;60(4):701-712. doi: 10.1111/head.13737. Epub 2020 Jan 27. Headache. 2020. PMID: 31985049 Free PMC article. Clinical Trial.
-
Vascular effects of 5-HT1B/1D-receptor agonists in patients with migraine headaches.Clin Pharmacol Ther. 2000 Oct;68(4):418-26. doi: 10.1067/mcp.2000.110502. Clin Pharmacol Ther. 2000. PMID: 11061582 Clinical Trial.
-
[Venous tonus-modifying effect, pharmacokinetics and undesired effects of dihydroergotamine].Z Gesamte Inn Med. 1984 Sep 1;39(17):417-28. Z Gesamte Inn Med. 1984. PMID: 6390997 Review. German.
Cited by
-
The Levels of Circulating Proangiogenic Factors in Migraineurs.Neuromolecular Med. 2017 Dec;19(4):510-517. doi: 10.1007/s12017-017-8465-7. Epub 2017 Sep 16. Neuromolecular Med. 2017. PMID: 28918499 Free PMC article.
-
Dihydroergotamine and its metabolite, 8'-hydroxy-dihydroergotamine, as 5-HT1A receptor agonists in the rat brain.Br J Pharmacol. 2003 May;139(2):424-34. doi: 10.1038/sj.bjp.0705258. Br J Pharmacol. 2003. PMID: 12770948 Free PMC article.
-
Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of the CGRP Binding Monoclonal Antibody LY2951742 (Galcanezumab) in Healthy Volunteers.Front Pharmacol. 2017 Oct 17;8:740. doi: 10.3389/fphar.2017.00740. eCollection 2017. Front Pharmacol. 2017. PMID: 29089894 Free PMC article.
-
Relationship of dihydroergotamine pharmacokinetics, clinical efficacy, and nausea-A narrative review.Headache. 2025 Mar;65(3):527-535. doi: 10.1111/head.14877. Epub 2024 Nov 27. Headache. 2025. PMID: 39601088 Free PMC article. Review.
References
-
- Silberstein SD, Young WB. Safety and efficacy of ergotamine tartrate and dihydroergotamine in the treatment of migraine and status migrainosus. Neurology. 1995;45:577–584. - PubMed
-
- Silberstein SD. The pharmacology of ergotamine and dihydroergotamine. Headache. 1997;37:S15–S25. - PubMed
-
- Clark BJ, Chu D, Aellig WH. Actions on the heart and circulation. In: Berde B, Schild HO, editors. Ergot alkaloids and related compounds. Berlin Heidelberg New York: Springer-Verlag; 1978. pp. 321–420.
-
- Muller-Schweinitzer E. What is known about the action of dihydroergotamine on the vasculature in man? Int J Clin Pharmacol Ther Toxicol. 1984;22:677–682. - PubMed
-
- Muller-Schweinitzer E, Rosenthaler J. Ex vivo studies after oral treatment of the beagle with dihydroergotamine. Eur J Pharmacol. 1983;89:1–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

