In vitro metabolism and drug interaction potential of a new highly potent anti-cytomegalovirus molecule, CMV423 (2-chloro 3-pyridine 3-yl 5,6,7,8-tetrahydroindolizine I-carboxamide)
- PMID: 11453890
- PMCID: PMC2014500
- DOI: 10.1046/j.0306-5251.2001.01413.x
In vitro metabolism and drug interaction potential of a new highly potent anti-cytomegalovirus molecule, CMV423 (2-chloro 3-pyridine 3-yl 5,6,7,8-tetrahydroindolizine I-carboxamide)
Abstract
Aims: To identify the enzymes involved in the metabolism of CMV423, a new anticytomegalovirus molecule, to evaluate its in vitro clearance and to investigate its potential involvement in drug/drug interactions that might occur in the clinic.
Methods: The enzymes involved in and the kinetics of CMV423 biotransformation were determined using pools of human liver subcellular fractions and heterologously expressed human cytochromes P450 (CYP) and FMO. The effect of CMV423 on CYP probe activities as well as on indinavir and AZT metabolism was determined, and 26 drugs were tested for their potential to inhibit or activate CMV423 metabolism.
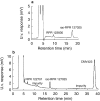
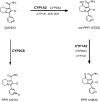
Results: CMV423 was oxidized by CYP and not by FMO or cytosolic enzymes. The Km values for 8-hydroxylation to rac-RPR 127025, an active metabolite, and subsequent ketone formation by human liver microsomes were 44 +/- 13 microM and 47 +/- 11 microM, respectively, with corresponding Vmax/Km ratios of 14 and 4 microl min(-1) nmol(-1) P450. Inhibition with selective CYP inhibitors indicated that CYP1A2 was the main isoform involved, with some participation from CYP3A. Expressed human CYP1A1, 1A2, 2C9, 3A4 and 2C8 catalysed rac-RPR 127025 formation with Km values of < 10 microM, 50 +/- 21 microM, 55 +/- 19 microM, circa 282 +/- 61 microM and circa 1450 microM, respectively. CYP1B1, 2A6, 2B6, 2C19, 2D6, 2E1 or 3A5 did not catalyse the reaction to any detectable extent. CYP1A1 and 3A4 also catalysed ketone formation from rac-RPR 127025. In human liver microsomes, CMV423 at 1 and 10 microM inhibited CYP1A2 activity up to 31% and 63%, respectively, CYP3A4 activity up to 40% (10 microM) and CYP2C9 activity by 35% (1 and 10 microM). No effect was observed on CYP2A6, 2D6 and 2E1 activities. CMV423 had no effect on indinavir and AZT metabolism. Amongst 26 drugs tested, none inhibited CMV423 metabolism in vitro at therapeutic concentrations.
Conclusions: CMV423 is mainly metabolized by CYP1A2 and 3A4. Its metabolism should not be saturable at the targeted therapeutic concentrations range (Cmax < 1 microM). CMV423 will probably affect CYP1A2 and 1A1 activities in vivo to some extent, but no other drug-drug interactions are expected.
Figures
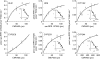
Similar articles
-
Kinetic characterization and identification of the enzymes responsible for the hepatic biotransformation of adinazolam and N-desmethyladinazolam in man.J Pharm Pharmacol. 1998 Mar;50(3):265-74. doi: 10.1111/j.2042-7158.1998.tb06859.x. J Pharm Pharmacol. 1998. PMID: 9600717
-
Biotransformation of parathion in human liver: participation of CYP3A4 and its inactivation during microsomal parathion oxidation.J Pharmacol Exp Ther. 1997 Feb;280(2):966-73. J Pharmacol Exp Ther. 1997. PMID: 9023313
-
Identification of CYP3A4 as the predominant isoform responsible for the metabolism of ambroxol in human liver microsomes.Xenobiotica. 2000 Jan;30(1):71-80. doi: 10.1080/004982500237839. Xenobiotica. 2000. PMID: 10659952
-
Cytochromes P450 and metabolism of xenobiotics.Cell Mol Life Sci. 2001 May;58(5-6):737-47. doi: 10.1007/pl00000897. Cell Mol Life Sci. 2001. PMID: 11437235 Free PMC article. Review.
-
Drug and diet interactions: avoiding therapeutic paralysis.J Clin Psychiatry. 1998;59 Suppl 16:31-9; discussion 40-2. J Clin Psychiatry. 1998. PMID: 9796864 Review.
Cited by
-
In vitro metabolism of bencycloquidium bromide and its inhibitory effects on human P450 isoenzymes: implication of CYP2D6, CYP2C19 and CYP3A4/5.Eur J Drug Metab Pharmacokinet. 2016 Feb;41(1):69-77. doi: 10.1007/s13318-014-0237-2. Epub 2014 Nov 26. Eur J Drug Metab Pharmacokinet. 2016. PMID: 25425116
References
-
- van der Meer J, Drew W, Bowden R, et al. Summary of the International Consensus Symposium on advances in the diagnosis, treatment and prophylaxis of cytomegalovirus infection. Antiviral Res. 1996;32:119–140. - PubMed
-
- Peck C, Temple R, Collins J. Understanding consequences of concurrent therapies. J Am Med Assoc. 1993;269:1550–1552. - PubMed
-
- Takahashi H, Kashima T, Kimura S, et al. Pharmacokinetic interaction between warfarin and a uricosuric agent, bucolome: application of in vitro approaches to predicting in vivo reduction of (S)-warfarin clearance. Drug Metab Dispos. 1999;27:1179–1186. - PubMed
-
- Sanderink G-J, Bournique B, Stevens J, Petry M, Martinet M. Involvement of human CYP1A isoenzymes in the metabolism and drug interactions of riluzole in vitro. J Pharmacol Exp Ther. 1997;282:1465–1472. - PubMed
-
- Strittmatter P, Fleming P, Connors M, Corcoran D. Purification of cytochrome b5. Meth Enzymol. 1978;52:97–101. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

