Systematic characterization of human CD8+ T cells with natural killer cell markers in comparison with natural killer cells and normal CD8+ T cells
- PMID: 11454057
- PMCID: PMC1783250
- DOI: 10.1046/j.1365-2567.2001.01248.x
Systematic characterization of human CD8+ T cells with natural killer cell markers in comparison with natural killer cells and normal CD8+ T cells
Abstract
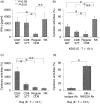
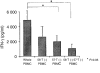
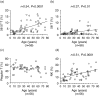
We investigated the function of CD56+ CD8+ T cells (CD56+ T cells) and CD56- CD57+ CD8+ T cells (CD57+ T cells; natural killer (NK)-type T cells) and compared them with those of normal CD56- CD57- CD8+ T cells (CD8+ T cells) and CD56+ NK cells from healthy volunteers. After the stimulation with immobilized anti-CD3 antibodies, both NK-type T cells produced much larger amounts of interferon-gamma (IFN-gamma) than CD8+ T cells. Both NK-type T cells also acquired a more potent cytotoxicity against NK-sensitive K562 cells than CD8+ T cells while only CD56+ T cells showed a potent cytotoxicity against NK-resistant Raji cells. After the stimulation with a combination of interleukin (IL)-2, IL-12 and IL-15, the IFN-gamma amounts produced were NK cells > or = CD56+ T cells > or = CD57+ T cells > CD8+ T cells. The cytotoxicities against K562 cells were NK cells > CD56+ T cells > or = CD57+ T cells > CD8+ T cells while cytotoxicities against Raji cells were CD56+ T cells > CD57+ T cells > or = CD8+ T cells > or = NK cells. However, the CD3-stimulated proliferation of both NK-type T cells was smaller than that of CD8+ T cells partly because NK-type T cells were susceptible to apoptosis. In addition to NK cells, NK-type T cells but not CD8+ T cells stimulated with cytokines, expressed cytoplasmic perforin and granzyme B. Furthermore, CD3-stimulated IFN-gamma production from peripheral blood mononuclear cells (PBMC) correlated with the proportions of CD57+ T cells in PBMC from donors. Our findings suggest that NK-type T cells play an important role in the T helper 1 responses and the immunological changes associated with ageing.
Figures






References
-
- Abo T, Balch CM. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1) J Immunol. 1981;127:1024–9. - PubMed
-
- Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986;136:4480–6. - PubMed
-
- Kruse J, Mailhammer R, Wernecke H, Faissner A, Sommer I, Goridis C, Schachner M. Neural cell adhesion molecules and myelin-associated glycoprotein share a common carbohydrate moiety recognized by monoclonal antibodies L2 and HNK-1. Nature. 1984;311:153–5. - PubMed
-
- Abo T, Watanabe H, Iiai T, et al. Extrathymic pathways of T-cell differentiation in the liver and other organs. Int Rev Immunol. 1994;11:61–102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

