Endocytosis and recycling of the complex between CD23 and HLA-DR in human B cells
- PMID: 11454061
- PMCID: PMC1783243
- DOI: 10.1046/j.1365-2567.2001.01238.x
Endocytosis and recycling of the complex between CD23 and HLA-DR in human B cells
Abstract
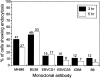
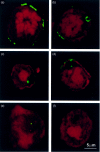
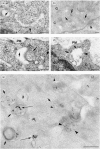
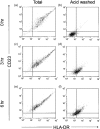
The presentation of extremely low doses of antigen to T cells is enhanced by immunoglobulin E (IgE)-dependent antigen focusing to CD23, the low-affinity receptor for IgE, expressed on activated B cells. CD23 contains a C-type lectin domain in its extracellular sequence and a targeting signal for coated pits, required for endocytosis, in its cytoplasmic sequence. CD23 is non-covalently associated with the major histocompatibility complex class II antigen, human leucocyte antigen HLA-DR, on the surface of human B cells, but the fate of this complex following endocytosis is unknown. To answer this question we have labelled these proteins on the surface of RPMI 8866 B cells and traced their route through the cytoplasm. Endocytosis mediated by anti-CD23 antibodies (BU38 and MHM6) led to the loss of CD23 from the cells. Endocytosis mediated by an antibody to HLA-DR (CR3/43) or an antigen-IgE complex (NP-BSA-anti-NP IgE), however, led to recycling of the HLA-DR-CD23 complex to the cell surface on a time scale (3-6 hr) consistent with the recycling of HLA-DR in antigen presentation. Along the latter pathway CD23 label was observed in cytoplasmic organelles that resembled the 'compartments for peptide loading' or 'class II vesicles' described by previous authors. Two features of the recycling process may contribute to the efficiency of antigen presentation. Peptide exchange may be facilitated by the proximity of HLA-DR and antigen in peptide loading compartments of the endosomal network. The return of CD23 with HLA-DR to the cell surface may then help to stabilize specific B-cell-T-cell interactions, contributing to T-cell activation.
Figures
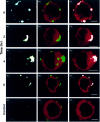
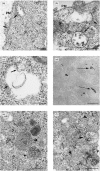
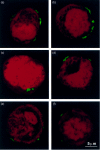
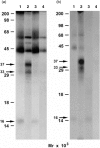
References
-
- Yokota A, Kikutani H, Tanaka T, Sato R, Barsumian EL, Suemura M, Kishimoto T. Two species of human Fcε receptor II (FcεRII/CD23): tissue-specific and IL-4 specific regulation of gene expression. Cell. 1988;55:611–18. - PubMed
-
- Yokota A, Yukawa K, Yamamoto A, Sugiyama K, Suemura M, Tashiro Y, Kishimoto T, Kikutani H. Two forms of the low-affinity Fc receptor for the IgE differentially mediate endocytosis and phagocytosis: Identification of the critical cytoplasmic domains. Proc Natl Acad Sci USA. 1992;89:5030–4. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

