Viruses and apoptosis
- PMID: 11454099
- PMCID: PMC2517702
- DOI: 10.1111/j.1365-2613.2001.iep0082-0065-x
Viruses and apoptosis
Abstract
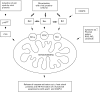
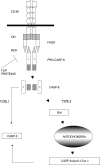
Apoptosis, or programmed cell death, is essential in development and homeostasis in multi-cellular organisms. It is also an important component of the cellular response to injury. Many cells undergo apoptosis in response to viral infection, with a consequent reduction in the release of progeny virus. Viruses have therefore evolved multiple distinct mechanisms for modulating host cell apoptosis. Viruses may interfere with either the highly conserved 'effector' mechanisms of programmed cell death or regulatory mechanisms specific to mammalian cells. In addition to conferring a selective advantage to the virus, the capacity to prevent apoptosis has an essential role in the transformation of the host cell by oncogenic viruses. This article provides a focussed review of apoptosis and illustrates how the study of viruses has informed our understanding of this process. Selected mechanisms by which viral gene products interfere with cell death are discussed in detail and used to illustrate the general principles of the interactions between viruses and apoptosis.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

