Loss of transforming growth factor beta signalling in the intestine contributes to tissue injury in inflammatory bowel disease
- PMID: 11454793
- PMCID: PMC1728415
- DOI: 10.1136/gut.49.2.190
Loss of transforming growth factor beta signalling in the intestine contributes to tissue injury in inflammatory bowel disease
Abstract
Background: Inflammatory bowel disease (IBD) is a chronic inflammation of the gastrointestinal tract caused by an abnormal and uncontrolled immune response to one or more normally occurring gut constituents.
Aim: Given the effects of transforming growth factor beta1 (TGF-beta1) on both the immune system and extracellular matrix, we postulated that alterations in TGF-beta signalling in intestinal epithelial cells may play an important role in the development of IBD.
Methods: TGF-beta signalling was inactivated in mouse intestine by expressing a dominant negative mutant form of the TGF-beta type II receptor under the control of the mouse intestinal trefoil peptide (ITF)/TFF3 promoter. Transgenic mice (ITF-dnRII) developed spontaneous colitis presenting with diarrhoea, haematochezia, and anal prolapse when not maintained under specific pathogen free (SPF) conditions. Under SPF conditions we induced colitis by mixing dextran sodium sulphate (DSS) in drinking water to examine the significance of loss of TGF-beta signalling in the pathogenesis of IBD.
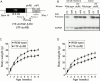
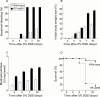
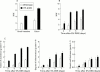
Results: Transgenic mice showed increased susceptibility to DSS induced IBD, and elicited increased expression of major histocompatibility complex class II, generation of autoantibodies against intestinal goblet cells, and increased activity of matrix metalloproteinase in intestinal epithelial cells compared with wild-type littermates challenged with DSS.
Conclusions: Deficiency of TGF-beta signalling specifically in the intestine contributes to the development of IBD. Maintenance of TGF-beta signalling may be important in regulating immune homeostasis in the intestine
Figures



Comment in
-
Signals on the immune tract.Gut. 2001 Aug;49(2):164-5. doi: 10.1136/gut.49.2.164. Gut. 2001. PMID: 11454787 Free PMC article. No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
