Interferon gamma inhibits growth of human pancreatic carcinoma cells via caspase-1 dependent induction of apoptosis
- PMID: 11454803
- PMCID: PMC1728385
- DOI: 10.1136/gut.49.2.251
Interferon gamma inhibits growth of human pancreatic carcinoma cells via caspase-1 dependent induction of apoptosis
Abstract
Background and aims: The poor prognosis of pancreatic cancer is partly due to resistance to a broad spectrum of apoptotic stimuli. To identify intact proapoptotic pathways of potential clinical relevance, we characterised the effects of interferon gamma (IFN-gamma) on growth and survival in human pancreatic cancer cells.
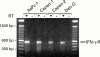
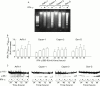
Methods: IFN-gamma receptor expression and signal transduction were examined by reverse transcriptase-polymerase chain reaction (RT-PCR), immunoprecipitation, western blot analysis, and transactivation assays. Effects on cell growth and survival were evaluated in terms of cell numbers, colony formation, cell cycle analysis, DNA fragmentation, and poly(ADP ribose) polymerase (PARP) cleavage.
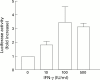
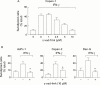
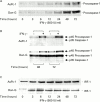
Results: All four pancreatic cancer cell lines examined expressed functional IFN-gamma receptors and downstream effectors, including the putative tumour suppressor interferon regulatory factor 1 (IRF-1). IFN-gamma treatment profoundly inhibited anchorage dependent and independent growth of pancreatic cancer cells. Cell cycle analyses revealed subdiploid cells suggesting apoptosis, which was confirmed by demonstration of DNA fragmentation and PARP cleavage. Time and dose dependency of apoptosis induction and growth inhibition correlated closely, identifying apoptosis as the main, if not exclusive, mechanism responsible for growth inhibition. Apoptosis was preceded by upregulation of procaspase-1 and accompanied by proteolytic activation. Furthermore, the caspase inhibitor z-vad-fmk completely prevented IFN-gamma mediated apoptosis.
Conclusions: These results identify an intact proapoptotic pathway in pancreatic cancer cells and suggest that IRF-1 and/or procaspase-1 may represent potential therapeutic targets to be further explored.
Figures




Similar articles
-
Interferon-gamma inhibits growth of human neuroendocrine carcinoma cells via induction of apoptosis.Int J Oncol. 2002 Nov;21(5):1133-40. doi: 10.3892/ijo.21.5.1133. Int J Oncol. 2002. PMID: 12370765
-
Interferon-gamma induces apoptosis of lens alphaTN4-1 cells and proteasome inhibition has an antiapoptotic effect.Invest Ophthalmol Vis Sci. 2004 Jan;45(1):222-9. doi: 10.1167/iovs.03-0571. Invest Ophthalmol Vis Sci. 2004. PMID: 14691177
-
Interferon regulatory factor-1 mediates interferon-gamma-induced apoptosis in ovarian carcinoma cells.J Cell Biochem. 2002;85(2):369-80. doi: 10.1002/jcb.10142. J Cell Biochem. 2002. PMID: 11948692
-
The two faces of interferon-γ in cancer.Clin Cancer Res. 2011 Oct 1;17(19):6118-24. doi: 10.1158/1078-0432.CCR-11-0482. Epub 2011 Jun 24. Clin Cancer Res. 2011. PMID: 21705455 Free PMC article. Review.
-
Regulation of apoptosis resistance and ontogeny of age-dependent diseases.Exp Gerontol. 1997 Jul-Oct;32(4-5):471-84. doi: 10.1016/s0531-5565(96)00156-8. Exp Gerontol. 1997. PMID: 9315450 Review.
Cited by
-
IRF-1 inhibits NF-κB activity, suppresses TRAF2 and cIAP1 and induces breast cancer cell specific growth inhibition.Cancer Biol Ther. 2015;16(7):1029-41. doi: 10.1080/15384047.2015.1046646. Cancer Biol Ther. 2015. PMID: 26011589 Free PMC article.
-
Wedelolactone, a naturally occurring coumestan, enhances interferon-γ signaling through inhibiting STAT1 protein dephosphorylation.J Biol Chem. 2013 May 17;288(20):14417-14427. doi: 10.1074/jbc.M112.442970. Epub 2013 Apr 11. J Biol Chem. 2013. PMID: 23580655 Free PMC article.
-
The inflammasome: an emerging therapeutic oncotarget for cancer prevention.Oncotarget. 2016 Aug 2;7(31):50766-50780. doi: 10.18632/oncotarget.9391. Oncotarget. 2016. PMID: 27206676 Free PMC article. Review.
-
Inetetamab, a novel anti-HER2 monoclonal antibody, exhibits potent synergistic anticancer effects with cisplatin by inducing pyroptosis in lung adenocarcinoma.Int J Biol Sci. 2023 Aug 6;19(13):4061-4081. doi: 10.7150/ijbs.82980. eCollection 2023. Int J Biol Sci. 2023. PMID: 37705753 Free PMC article.
-
Substantially reduced expression of PIAS1 is associated with colon cancer development.J Cancer Res Clin Oncol. 2009 Sep;135(9):1287-91. doi: 10.1007/s00432-009-0570-z. Epub 2009 Mar 15. J Cancer Res Clin Oncol. 2009. PMID: 19288270 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
