Local Ca(2+) transients and distribution of BK channels and ryanodine receptors in smooth muscle cells of guinea-pig vas deferens and urinary bladder
- PMID: 11454953
- PMCID: PMC2278703
- DOI: 10.1111/j.1469-7793.2001.t01-3-00313.x
Local Ca(2+) transients and distribution of BK channels and ryanodine receptors in smooth muscle cells of guinea-pig vas deferens and urinary bladder
Abstract
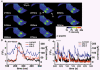
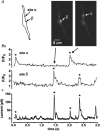
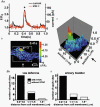
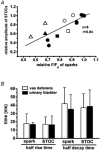
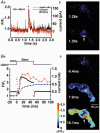
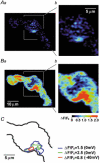
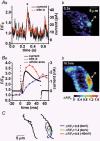
1. The relationship between Ca(2+) sparks spontaneously occurring at rest and local Ca(2+) transients elicited by depolarization was analysed using two-dimensional confocal Ca(2+) images of single smooth muscle cells isolated from guinea-pig vas deferens and urinary bladder. The current activation by these Ca(2+) events was also recorded simultaneously under whole-cell voltage clamp. 2. Spontaneous transient outward currents (STOCs) and Ca(2+) sparks were simultaneously detected at -40 mV in approximately 50 % of myocytes of either type. Ca(2+) sparks and corresponding STOCs occurred repetitively in several discrete sites in the subplasmalemmal area. Large conductance Ca(2+)-dependent K(+) (BK) channel density in the plasmalemma near the Ca(2+) spark sites generating STOCs was calculated to be 21 channels microm(-2). 3. When myocytes were depolarized from -60 to 0 mV, several local Ca(2+) transients were elicited within 20 ms in exactly the same peripheral sites where sparks occurred at rest. The local Ca(2+) transients often lasted over 300 ms and spread into other areas. The appearance of local Ca(2+) transients occurred synchronously with the activation of Ca(2+)-dependent K(+) current (I(K,Ca)). 4. Immunofluorescence staining of the BK channel alpha-subunit (BKalpha) revealed a spot-like pattern on the plasmalemma, in contrast to the uniform staining of voltage-dependent Ca(2+) channel alpha1C subunits along the plasmalemma. Ryanodine receptor (RyR) immunostaining also suggested punctate localization predominantly in the periphery. Double staining of BKalpha and RyRs revealed spot-like co-localization on/beneath the plasmalemma. 5. Using pipettes of relatively low resistance, inside-out patches that included both clustered BK channels at a density of over 20 channels microm(-2) and functional Ca(2+) storage sites were obtained at a low probability of approximately 5%. The averaged BK channel density was 3-4 channels microm(-2) in both types of myocyte. 6. These results support the idea that a limited number of discrete sarcoplasmic reticulum (SR) fragments in the subplasmalemmal area play key roles in the control of BK channel activity in two ways: (i) by generating Ca(2+) sparks at rest to activate STOCs and (ii) by generating Ca(2+) transients presumably triggered by sparks during an action potential to activate a large I(K,Ca) and also induce a contraction. BK channels and RyRs may co-localize densely at the junctional areas of plasmalemma and SR fragments, where Ca(2+) sparks occur to elicit STOCs.
Figures
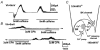
Similar articles
-
Differential regulation of SK and BK channels by Ca(2+) signals from Ca(2+) channels and ryanodine receptors in guinea-pig urinary bladder myocytes.J Physiol. 2002 Jun 1;541(Pt 2):483-92. doi: 10.1113/jphysiol.2002.017707. J Physiol. 2002. PMID: 12042353 Free PMC article.
-
Ca(2+) sparks and BK currents in gallbladder myocytes: role in CCK-induced response.Am J Physiol Gastrointest Liver Physiol. 2002 Jan;282(1):G165-74. doi: 10.1152/ajpgi.00326.2001. Am J Physiol Gastrointest Liver Physiol. 2002. PMID: 11751170
-
A close association of RyRs with highly dense clusters of Ca2+-activated Cl- channels underlies the activation of STICs by Ca2+ sparks in mouse airway smooth muscle.J Gen Physiol. 2008 Jul;132(1):145-60. doi: 10.1085/jgp.200709933. J Gen Physiol. 2008. PMID: 18591421 Free PMC article.
-
Signaling between SR and plasmalemma in smooth muscle: sparks and the activation of Ca2+-sensitive ion channels.Cell Calcium. 2003 Sep;34(3):211-29. doi: 10.1016/s0143-4160(03)00124-6. Cell Calcium. 2003. PMID: 12887969 Review.
-
Sarcoplasmic reticulum and membrane currents.Novartis Found Symp. 2002;246:189-203; discussion 203-7, 221-7. Novartis Found Symp. 2002. PMID: 12164309 Review.
Cited by
-
Multidimensional detection and analysis of Ca2+ sparks in cardiac myocytes.Biophys J. 2007 Jun 15;92(12):4433-43. doi: 10.1529/biophysj.106.089359. Epub 2007 Mar 16. Biophys J. 2007. PMID: 17369419 Free PMC article.
-
A junctophilin-caveolin interaction enables efficient coupling between ryanodine receptors and BKCa channels in the Ca2+ microdomain of vascular smooth muscle.J Biol Chem. 2019 Aug 30;294(35):13093-13105. doi: 10.1074/jbc.RA119.008342. Epub 2019 Jul 15. J Biol Chem. 2019. PMID: 31308177 Free PMC article.
-
Calcium mobilization and spontaneous transient outward current characteristics upon agonist activation of P2Y2 receptors in smooth muscle cells.Biophys J. 2005 Mar;88(3):1507-23. doi: 10.1529/biophysj.104.043976. Epub 2004 Nov 19. Biophys J. 2005. PMID: 15556987 Free PMC article.
-
Relationship between Ca2+ sparklets and sarcoplasmic reticulum Ca2+ load and release in rat cerebral arterial smooth muscle.Am J Physiol Heart Circ Physiol. 2011 Dec;301(6):H2285-94. doi: 10.1152/ajpheart.00488.2011. Epub 2011 Oct 7. Am J Physiol Heart Circ Physiol. 2011. PMID: 21984539 Free PMC article.
-
Spontaneous transient outward currents arise from microdomains where BK channels are exposed to a mean Ca(2+) concentration on the order of 10 microM during a Ca(2+) spark.J Gen Physiol. 2002 Jul;120(1):15-27. doi: 10.1085/jgp.20028571. J Gen Physiol. 2002. PMID: 12084772 Free PMC article.
References
-
- Arnaudeau S, Boittin FX, Macrez N, Lavie JL, Mironneau C, Mironneau J. L-type and Ca2+ release channel-dependent hierarchical Ca2+ signalling in rat portal vein myocytes. Cell Calcium. 1997;22:399–411. - PubMed
-
- Bolton TB, Imaizumi Y. Spontaneous transient outward currents in smooth muscle cells. Cell Calcium. 1996;20:141–152. - PubMed
-
- Bolton TB, Prestwich SA, Zholos AV, Gordienko DV. Excitation-contraction coupling in gastrointestinal and other smooth muscles. Annual Review of Physiology. 1999;61:85–115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

