Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues
- PMID: 11454958
- PMCID: PMC2278702
- DOI: 10.1111/j.1469-7793.2001.00381.x
Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues
Abstract
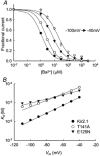
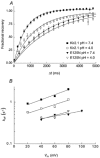
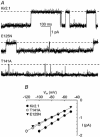
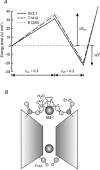
1. The block of the IRK1/Kir2.1 inwardly rectifying K+ channel by a Ba(2+) ion is highly voltage dependent, where the ion binds approximately half-way within the membrane electrical field. The mechanism by which two distinct mutations, E125N and T141A, affect Ba(2+) block of Kir2.1 was investigated using heterologous expression in Xenopus oocytes. 2. Analysis of the blocking kinetics showed that E125 and T141 affect the entry and binding of Ba(2+) to the channel, respectively. Replacing the glutamate at position 125 with an asparagine greatly decreased the rate at which the Ba(2+) ions enter and leave the pore. In contrast, replacing the polar threonine at position 141 with an alanine affected the entry rate of the Ba(2+) ions while leaving the exit rate unchanged. 3. Acidification of the extracellular solution slowed the exit rate of the Ba(2+) from the wild-type channel, but had no such effect on the Kir2.1(E125N) mutant. 4. These results thus reveal two unique roles for the amino acids at positions 125 and 141 in aiding the interaction of Ba(2+) with the channel. Their possible roles in K+ permeation are discussed.
Figures




References
-
- Biermans G, Vereecke J, Carmeliet E. The mechanism of the inactivation of the inward-rectifying K current during hyperpolarizing steps in guinea-pig ventricular myocytes. Pflügers Archiv. 1987;410:604–613. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

