Modulation of hypoglossal motoneuron excitability by NK1 receptor activation in neonatal mice in vitro
- PMID: 11454963
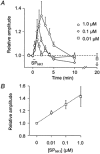
- PMCID: PMC2278713
- DOI: 10.1111/j.1469-7793.2001.00447.x
Modulation of hypoglossal motoneuron excitability by NK1 receptor activation in neonatal mice in vitro
Abstract
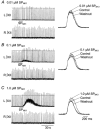
1. The effects of substance P (SP), acting at NK1 receptors, on the excitability and inspiratory activity of hypoglossal (XII) motoneurons (MNs) were investigated using rhythmically active medullary-slice preparations from neonatal mice (postnatal day 0-3). 2. Local application of the NK1 agonist [SAR(9),Met (O(2))(11)]-SP (SP(NK1)) produced a dose-dependent, spantide- (a non-specific NK receptor antagonist) and GR82334-(an NK1 antagonist) sensitive increase in inspiratory burst amplitude recorded from XII nerves. 3. Under current clamp, SP(NK1) significantly depolarized XII MNs, potentiated repetitive firing responses to injected currents and produced a leftward shift in the firing frequency-current relationships without affecting slope. 4. Under voltage clamp, SP(NK1) evoked an inward current and increased input resistance, but had no effect on inspiratory synaptic currents. SP(NK1) currents persisted in the presence of TTX, were GR82334 sensitive, were reduced with hyperpolarization and reversed near the expected E(K). 5. Effects of the alpha(1)-noradrenergic receptor agonist phenylephrine (PE) on repetitive firing behaviour were virtually identical to those of SP(NK1). Moreover, SP(NK1) currents were completely occluded by PE, suggesting that common intracellular pathways mediate the actions of NK1 and alpha(1)-noradrenergic receptors. In spite of the similar actions of SP(NK1) and PE on XII MN responses to somally injected current, alpha(1)-noradrenergic receptor activation potentiated inspiratory synaptic currents and was more than twice as effective in potentiating XII nerve inspiratory burst amplitude. 6. GR82334 reduced XII nerve inspiratory burst amplitude and generated a small outward current in XII MNs. These observations, together with the first immunohistochemical evidence in the newborn for SP immunopositive terminals in the vicinity of SP(NK1)-sensitive inspiratory XII MNs, support the endogenous modulation of XII MN excitability by SP. 7. In contrast to phrenic MNs (Ptak et al. 2000), blocking NMDA receptors with AP5 had no effect on the modulation of XII nerve activity by SP(NK1). 8. In conclusion, SP(NK1) modulates XII motoneuron responses to inspiratory drive primarily through inhibition of a resting, postsynaptic K+ leak conductance. The results establish the functional significance of SP in controlling upper airway tone during early postnatal life and indicate differential modulation of motoneurons controlling airway and pump muscles by SP.
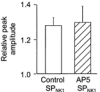
Figures

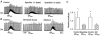






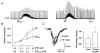

Similar articles
-
Substance P modulation of hypoglossal motoneuron excitability during development: changing balance between conductances.J Neurophysiol. 2010 Aug;104(2):854-72. doi: 10.1152/jn.00016.2010. Epub 2010 Jun 10. J Neurophysiol. 2010. PMID: 20538779
-
Noradrenergic modulation of XII motoneuron inspiratory activity does not involve alpha2-receptor inhibition of the Ih current or presynaptic glutamate release.J Appl Physiol (1985). 2005 Apr;98(4):1297-308. doi: 10.1152/japplphysiol.00977.2004. Epub 2004 Dec 3. J Appl Physiol (1985). 2005. PMID: 15579572
-
Cellular and synaptic effect of substance P on neonatal phrenic motoneurons.Eur J Neurosci. 2000 Jan;12(1):126-38. doi: 10.1046/j.1460-9568.2000.00886.x. Eur J Neurosci. 2000. PMID: 10651867
-
Synaptic control of motoneuron excitability in rodents: from months to milliseconds.Clin Exp Pharmacol Physiol. 2000 Jan-Feb;27(1-2):120-5. doi: 10.1046/j.1440-1681.2000.03202.x. Clin Exp Pharmacol Physiol. 2000. PMID: 10696540 Review.
-
Noradrenergic modulation of hypoglossal motoneuron excitability: developmental and putative state-dependent mechanisms.Arch Ital Biol. 2011 Dec 1;149(4):426-53. doi: 10.4449/aib.v149i4.1271. Arch Ital Biol. 2011. PMID: 22205594 Review.
Cited by
-
Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network.J Neurosci. 2004 Aug 25;24(34):7549-56. doi: 10.1523/JNEUROSCI.1871-04.2004. J Neurosci. 2004. PMID: 15329402 Free PMC article.
-
Substance P/Neurokinin 1 and Trigeminal System: A Possible Link to the Pathogenesis in Sudden Perinatal Deaths.Front Neurol. 2017 Mar 13;8:82. doi: 10.3389/fneur.2017.00082. eCollection 2017. Front Neurol. 2017. PMID: 28348544 Free PMC article. Review.
-
P2Y1 receptor-mediated potentiation of inspiratory motor output in neonatal rat in vitro.J Physiol. 2014 Jul 15;592(14):3089-111. doi: 10.1113/jphysiol.2013.268136. Epub 2014 May 30. J Physiol. 2014. PMID: 24879869 Free PMC article.
-
Neurokinin-1 receptor activation in globus pallidus.Front Neurosci. 2009 Oct 26;3:58. doi: 10.3389/neuro.23.002.2009. eCollection 2009. Front Neurosci. 2009. PMID: 20582283 Free PMC article.
-
Pathophysiology of sleep apnea.Physiol Rev. 2010 Jan;90(1):47-112. doi: 10.1152/physrev.00043.2008. Physiol Rev. 2010. PMID: 20086074 Free PMC article. Review.
References
-
- Abrahams TP, Hornby PJ, Walton DP, Taveira Dasilva AM, Gillis RA. An excitatory amino acid(s) in the ventrolateral medulla is (are) required for breathing to occur in the anesthetized cat. Journal of Pharmacology and Experimental Therapeutics. 1991;259:1388–1395. - PubMed
-
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Progress in Neurobiology. 1999;59:583–634. - PubMed
-
- Baranauskas G, Nistri A. Effects of RP 67580 on substance P-elicited responses and postsynaptic potentials of motoneurones of the rat isolated spinal cord. Peptides. 1995;16:357–359. - PubMed
-
- Bartlett DJ, Leiter JC, Knuth SL. Control and actions of the genioglossus muscle. In: Suratt FG, Remmers JE, editors. Sleep and Respiration. New York: Wiley-Liss; 1990. pp. 99–108. - PubMed
-
- Beresford IJ, Ireland SJ, Stables J, Hagan RM. Ontogeny and characterization of [125I]Bolton Hunter-eledoisin binding sites in rat spinal cord by quantitative autoradiography. Neuroscience. 1992;46:225–232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

