Medullary respiratory neurones and control of laryngeal motoneurones during fictive eupnoea and cough in the cat
- PMID: 11454973
- PMCID: PMC2278720
- DOI: 10.1111/j.1469-7793.2001.t01-1-00565.x
Medullary respiratory neurones and control of laryngeal motoneurones during fictive eupnoea and cough in the cat
Abstract
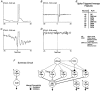
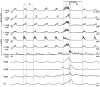
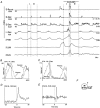
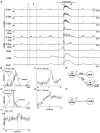
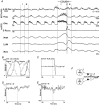
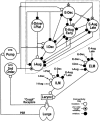
1. This study addressed the hypothesis that ventrolateral medullary respiratory neurones participate in the control of laryngeal motoneurones during both eupnoea and coughing. 2. Data were obtained from 28 mid-collicular decerebrated, artificially ventilated cats. Cough-like motor patterns (fictive cough) in phrenic, lumbar and recurrent laryngeal nerves were elicited by mechanical stimulation of the intrathoracic trachea. Microelectrode arrays were used to monitor simultaneously several neurones in the ventral respiratory group, including the Bötzinger and pre-Bötzinger complexes. Spike trains were evaluated for responses during fictive cough and evidence of functional connectivity with spike-triggered averages of efferent recurrent laryngeal nerve activity. 3. Primary features were observed in averages triggered by 94 of 332 (28 %) neurones. An offset biphasic wave with a positive time lag was present in the unrectified average for 10 inspiratory and 13 expiratory neurones. These trigger neurones were respectively identified as inspiratory laryngeal motoneurones with augmenting, decrementing, plateau and "other" discharge patterns, and expiratory laryngeal motoneurones with decrementing firing patterns. 4. Rectified averages triggered by inspiratory neurones included 37 offset peaks, 11 central peaks and one offset trough. Averages triggered by expiratory neurones had 12 offset peaks, six central peaks and four offset troughs. Relationships inferred from these features included premotor actions of inspiratory neurones with augmenting, decrementing, plateau and "other" patterns on inspiratory laryngeal motoneurones, and premotor actions of decrementing and "other" expiratory neurones on expiratory laryngeal motoneurones. Corresponding changes in neuronal firing patterns during fictive cough supported these inferences. 5. The data confirm and extend previous results on the control of laryngeal motoneurones during eupnoea and support the hypothesis that the same premotor neurones help to shape motoneurone firing patterns during both eupnoea and coughing.
Figures

References
-
- Balis UJ, Morris KF, Koleski J, Lindsey BG. Simulation of ventrolateral medullary neural network for respiratory rhythmogenesis inferred from spike train cross-correlation. Biological Cybernetics. 1994;70:311–327. - PubMed
-
- Barillot JC, Bianchi AL. Activité des motoneurones laryngés pendant le réflexe de Hering-Breuer. Journal de Physiologie. 1971;63:783–792. - PubMed
-
- Barillot JC, Bianchi AL, Gogan P. Laryngeal expiratory motoneurones: morphology and electrophysiological evidence of separate sites for excitatory and inhibitory synaptic events. Neuroscience Letters. 1984;47:107–112. - PubMed
-
- Barillot JC, Dussardier M. Activité des motoneurones laryngés expiratoires. Journal de Physiologie. 1976;72:311–343. - PubMed
-
- Barillot JC, Grélot L, Reddad S, Bianchi AL. Discharge patterns of laryngeal motoneurones in the cat: an intracellular study. Brain Research. 1990;509:99–106. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

