Progressive atrioventricular conduction defects and heart failure in mice expressing a mutant Csx/Nkx2.5 homeoprotein
- PMID: 11457872
- PMCID: PMC203028
- DOI: 10.1172/JCI12694
Progressive atrioventricular conduction defects and heart failure in mice expressing a mutant Csx/Nkx2.5 homeoprotein
Abstract
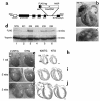
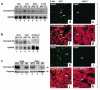
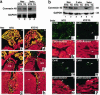
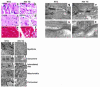
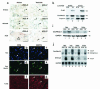
A DNA nonbinding mutant of the NK2 class homeoprotein Nkx2.5 dominantly inhibits cardiogenesis in Xenopus embryos, causing a small heart to develop or blocking heart formation entirely. Recently, ten heterozygous CSX/NKX2.5 homeoprotein mutations were identified in patients with congenital atrioventricular (AV) conduction defects. All four missense mutations identified in the human homeodomain led to markedly reduced DNA binding. To examine the effect of a DNA binding-impaired mutant of mouse Csx/Nkx2.5 in the embryonic heart, we generated transgenic mice expressing one such allele, I183P, under the beta-myosin heavy chain promoter. Unexpectedly, transgenic mice were born apparently normal, but the accumulation of Csx/Nkx2.5(I183P) mutant protein in the embryo, neonate, and adult myocardium resulted in progressive and profound cardiac conduction defects and heart failure. P-R prolongation observed at 2 weeks of age rapidly progressed into complete AV block as early as 4 weeks of age. Expression of connexins 40 and 43 was dramatically decreased in the transgenic heart, which may contribute to the conduction defects in the transgenic mice. This transgenic mouse model may be useful in the study of the pathogenesis of cardiac dysfunction associated with CSX/NKX2.5 mutations in humans.
Figures
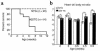

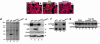

Similar articles
-
Developmentally modulated cardiac conduction failure in transgenic mice with fetal or postnatal overexpression of DNA nonbinding mutant Nkx2.5.J Cardiovasc Electrophysiol. 2002 Jul;13(7):682-8. doi: 10.1046/j.1540-8167.2002.00682.x. J Cardiovasc Electrophysiol. 2002. PMID: 12139292
-
Nkx2.5 homeoprotein regulates expression of gap junction protein connexin 43 and sarcomere organization in postnatal cardiomyocytes.J Mol Cell Cardiol. 2003 Mar;35(3):243-56. doi: 10.1016/s0022-2828(03)00002-6. J Mol Cell Cardiol. 2003. PMID: 12676539
-
Cardiac electrophysiological phenotypes in postnatal expression of Nkx2.5 transgenic mice.Genesis. 2003 Nov;37(3):144-50. doi: 10.1002/gene.10236. Genesis. 2003. PMID: 14595838
-
Cardiac transcription factor Csx/Nkx2-5: Its role in cardiac development and diseases.Pharmacol Ther. 2005 Aug;107(2):252-68. doi: 10.1016/j.pharmthera.2005.03.005. Pharmacol Ther. 2005. PMID: 15925411 Review.
-
Function follows form: cardiac conduction system defects in Nkx2-5 mutation.Anat Rec A Discov Mol Cell Evol Biol. 2004 Oct;280(2):966-72. doi: 10.1002/ar.a.20102. Anat Rec A Discov Mol Cell Evol Biol. 2004. PMID: 15368343 Review.
Cited by
-
Compound loss of muscleblind-like function in myotonic dystrophy.EMBO Mol Med. 2013 Dec;5(12):1887-900. doi: 10.1002/emmm.201303275. Epub 2013 Oct 8. EMBO Mol Med. 2013. PMID: 24293317 Free PMC article.
-
Activation of Notch signaling pathway precedes heart regeneration in zebrafish.Proc Natl Acad Sci U S A. 2003 Sep 30;100 Suppl 1(Suppl 1):11889-95. doi: 10.1073/pnas.1834204100. Epub 2003 Aug 8. Proc Natl Acad Sci U S A. 2003. PMID: 12909711 Free PMC article.
-
Heart failure in congenital heart disease: the role of genes and hemodynamics.Pflugers Arch. 2014 Jun;466(6):1025-35. doi: 10.1007/s00424-014-1447-9. Epub 2014 Feb 1. Pflugers Arch. 2014. PMID: 24488006 Review.
-
MURC, a muscle-restricted coiled-coil protein that modulates the Rho/ROCK pathway, induces cardiac dysfunction and conduction disturbance.Mol Cell Biol. 2008 May;28(10):3424-36. doi: 10.1128/MCB.02186-07. Epub 2008 Mar 10. Mol Cell Biol. 2008. PMID: 18332105 Free PMC article.
-
Characterization of embryonic cardiac pacemaker and atrioventricular conduction physiology in Xenopus laevis using noninvasive imaging.Am J Physiol Heart Circ Physiol. 2004 Jun;286(6):H2035-41. doi: 10.1152/ajpheart.00807.2003. Am J Physiol Heart Circ Physiol. 2004. PMID: 15148055 Free PMC article.
References
-
- Bodmer R. The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development. 1993;118:719–729. - PubMed
-
- Azpiazu N, Frasch M. Tinman and bagpipe: two homeo box genes that determine cell fates in the dorsal mesoderm of Drosophila. Genes Dev. 1993;7:1325–1340. - PubMed
-
- Lints TJ, Parsons LM, Hartley L, Lyons I, Harvey RP. Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development. 1993;119:419–431. - PubMed
-
- Tonissen KF, Drysdale TA, Lints TJ, Harvey RP, Krieg PA. XNkx-2.5, a Xenopus gene related to Nkx-2.5 and tinman: evidence for a conserved role in cardiac development. Dev Biol. 1994;162:325–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

