A family with complement factor D deficiency
- PMID: 11457876
- PMCID: PMC203023
- DOI: 10.1172/JCI12023
A family with complement factor D deficiency
Abstract
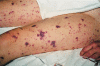
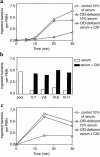
A complement factor D deficiency was found in a young woman who had experienced a serious Neisseria meningitidis infection, in a deceased family member with a history of meningitis, and in three relatives without a history of serious infections. The patient and these three relatives showed a normal activity of the classical complement pathway, but a very low activity of the alternative complement pathway and a very low capacity to opsonize Escherichia coli and N. meningitidis (isolated from the patient) for phagocytosis by normal human neutrophils. The alternative pathway-dependent hemolytic activity and the opsonizing capacity of these sera were restored by addition of purified factor D. The family had a high degree of consanguinity, and several other family members exhibited decreased levels of factor D. The gene encoding factor D was found to contain a point mutation that changed the TCG codon for serine 42 into a TAG stop codon. This mutation was found in both alleles of the five completely factor D-deficient family members and in one allele of 21 other members of the same family who had decreased or low-normal factor D levels in their serum. The gene sequence of the signal peptide of human factor D was also identified. Our report is the first, to our knowledge, to document a Factor D gene mutation. The mode of inheritance of factor D deficiency is autosomal recessive, in accordance with the localization of the Factor D gene on chromosome 19. Increased susceptibility for infections in individuals with a partial factor D deficiency is unlikely.
Figures


References
-
- White RT, et al. Human adipsin is identical to complement factor D and is expressed at high levels in adipose tissue. J Biol Chem. 1992;267:9210–9213. - PubMed
-
- Figueroa J, Andreoni J, Densen P. Complement deficiency states and meningococcal disease. Immunol Res. 1993;12:295–311. - PubMed
-
- Meyer, M.M. 1961. In Experimental immunochemistry. 2nd edition. Kabat, E.A., and Meyer, M.M., editors. Charles C. Thomas. Springfield, Illinois, USA. 133–240
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

