Retention of CXCR4 in the endoplasmic reticulum blocks dissemination of a T cell hybridoma
- PMID: 11457880
- PMCID: PMC203019
- DOI: 10.1172/JCI11330
Retention of CXCR4 in the endoplasmic reticulum blocks dissemination of a T cell hybridoma
Abstract
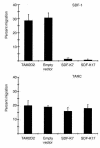
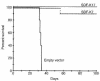
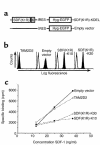
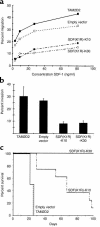
The dissemination of T cell hybridomas to multiple nonhematopoietic tissues is blocked by pertussis toxin, suggesting the involvement of a chemokine. To study whether this chemokine is SDF-1, we employed a strategy proposed previously for gene therapy of AIDS, whereby the SDF-1 receptor CXCR4 (also a coreceptor for HIV) is retained in the endoplasmic reticulum (ER) and fails to reach the cell surface. We transfected SDF-1, carrying an ER retention sequence, into a T cell hybridoma. This altered chemokine is retained in the ER, where it binds CXCR4 and prevents the latter protein from reaching the surface. These cells failed to migrate toward SDF-1 or to invade fibroblast monolayers, although they could still migrate toward thymus and activation-regulated chemokine (TARC) and invade TARC-treated monolayers. Furthermore, the ability of the transfected cells to disseminate to multiple organs upon intravenous injection into mice was abolished. This dissemination reflects the in vivo migration patterns of activated and memory T cells into nonhematopoietic tissues, which is thus likely to depend on CXCR4. Attempts to block CXCR4 function as a therapy for AIDS may affect this migration with consequences for T cell function. Our results also suggest a decisive role for CXCR4 in the dissemination of hematopoietic malignancies expressing this receptor.
Figures


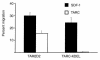
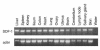
Similar articles
-
The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases.Cancer Res. 2003 Jul 1;63(13):3833-9. Cancer Res. 2003. PMID: 12839981
-
Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis.Stem Cells. 2005 Aug;23(7):879-94. doi: 10.1634/stemcells.2004-0342. Epub 2005 May 11. Stem Cells. 2005. PMID: 15888687 Review.
-
Phenotypic knockout of CXCR4 by a novel recombinant protein TAT/54R/KDEL inhibits tumors metastasis.Mol Cancer Res. 2009 Oct;7(10):1613-21. doi: 10.1158/1541-7786.MCR-09-0078. Epub 2009 Oct 13. Mol Cancer Res. 2009. PMID: 19825996
-
Intrakines--evidence for a trans-cellular mechanism of action.Mol Ther. 2000 Feb;1(2):165-70. doi: 10.1006/mthe.2000.0026. Mol Ther. 2000. PMID: 10933927
-
Stromal cell-derived factor-1 and CXCR4 receptor interaction in tumor growth and metastasis of breast cancer.Biomed Pharmacother. 2006 Jul;60(6):273-6. doi: 10.1016/j.biopha.2006.06.004. Epub 2006 Jun 28. Biomed Pharmacother. 2006. PMID: 16828253 Review.
Cited by
-
Construction of a CXCL12-KDEL fusion gene to inhibit head and neck squamous cell carcinoma metastasis by intracellular sequestration of CXCR4.Biomed Res Int. 2015;2015:195828. doi: 10.1155/2015/195828. Epub 2015 Mar 19. Biomed Res Int. 2015. PMID: 25866764 Free PMC article.
-
Expression of CXCR4 and non-small cell lung cancer prognosis: a meta-analysis.Int J Clin Exp Med. 2015 May 15;8(5):7435-45. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26221287 Free PMC article.
-
Effect of the chemokine receptor CXCR7 on proliferation of carcinoma cells in vitro and in vivo.Br J Cancer. 2008 Nov 4;99(9):1493-501. doi: 10.1038/sj.bjc.6604727. Epub 2008 Oct 14. Br J Cancer. 2008. Retraction in: Br J Cancer. 2011 Jan 4;104(1):227. doi: 10.1038/sj.bjc.6606002. PMID: 18854833 Free PMC article. Retracted.
-
Targeting CXCR4 with cell-penetrating pepducins in lymphoma and lymphocytic leukemia.Blood. 2012 Feb 16;119(7):1717-25. doi: 10.1182/blood-2011-04-347518. Epub 2011 Dec 20. Blood. 2012. PMID: 22186993 Free PMC article.
-
CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion.J Mol Histol. 2004 Mar;35(3):233-45. doi: 10.1023/b:hijo.0000032355.66152.b8. J Mol Histol. 2004. PMID: 15339043 Review.
References
-
- Roos E, La Riviere G, Collard JG, Stukart MJ, De Baetselier P. Invasiveness of T-cell hybridomas in vitro and their metastatic potential in vivo. Cancer Res. 1985;45:6238–6243. - PubMed
-
- La Rivière G, Gebbinck JW, Schipper CA, Mooi WJ, Roos E. In vitro invasiveness of CTL clones and in vivo dissemination of CTL hybridomas. J Leukoc Biol. 1993;53:381–389. - PubMed
-
- Driessens MHE, van Rijthoven EAM, La Rivière G, Roos E. Expression of pertussis toxin adenosine diphosphate-ribosyltransferase in a T-cell hybridoma reduces metastatic capacity. Blood. 1996;88:3116–3123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

