Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery
- PMID: 11457881
- PMCID: PMC203029
- DOI: 10.1172/JCI12761
Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery
Abstract
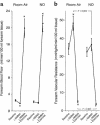
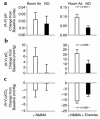
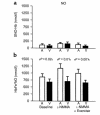
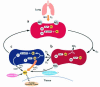
Nitric oxide (NO) may be stabilized by binding to hemoglobin, by nitrosating thiol-containing plasma molecules, or by conversion to nitrite, all reactions potentially preserving its bioactivity in blood. Here we examined the contribution of blood-transported NO to regional vascular tone in humans before and during NO inhalation. While breathing room air and then room air with NO at 80 parts per million, forearm blood flow was measured in 16 subjects at rest and after blockade of forearm NO synthesis with N(G)-monomethyl-L-arginine (L-NMMA) followed by forearm exercise stress. L-NMMA reduced blood flow by 25% and increased resistance by 50%, an effect that was blocked by NO inhalation. With NO inhalation, resistance was significantly lower during L-NMMA infusion, both at rest and during repetitive hand-grip exercise. S-nitrosohemoglobin and plasma S-nitrosothiols did not change with NO inhalation. Arterial nitrite levels increased by 11% and arterial nitrosyl(heme)hemoglobin levels increased tenfold to the micromolar range, and both measures were consistently higher in the arterial than in venous blood. S-nitrosohemoglobin levels were in the nanomolar range, with no significant artery-to-vein gradients. These results indicate that inhaled NO during blockade of regional NO synthesis can supply intravascular NO to maintain normal vascular function. This effect may have application for the treatment of diseases characterized by endothelial dysfunction.
Figures


References
-
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. - PubMed
-
- Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987;61:866–879. - PubMed
-
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. - PubMed
-
- Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

