The experimental herbicide CGA 325'615 inhibits synthesis of crystalline cellulose and causes accumulation of non-crystalline beta-1,4-glucan associated with CesA protein
- PMID: 11457949
- PMCID: PMC116455
- DOI: 10.1104/pp.126.3.981
The experimental herbicide CGA 325'615 inhibits synthesis of crystalline cellulose and causes accumulation of non-crystalline beta-1,4-glucan associated with CesA protein
Abstract
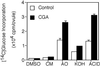
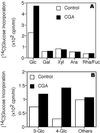
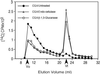
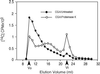
Developing cotton (Gossypium hirsutum) fibers, cultured in vitro with their associated ovules, were used to compare the effects of two herbicides that inhibit cellulose synthesis: 2,6-dichlorobenzonitrile (DCB) and an experimental thiatriazine-based herbicide, CGA 325'615. CGA 325'615 in nanomolar concentrations or DCB in micromolar concentrations causes inhibition of synthesis of crystalline cellulose. Unlike DCB, CGA 325'615 also causes concomitant accumulation of non-crystalline beta-1,4-glucan that can be at least partially solubilized from fiber walls with ammonium oxalate. The unusual solubility of this accumulated glucan may be explained by its strong association with protein. Treatment of the glucan fraction with protease changes its size distribution and leads to precipitation of the glucan. Treatment of the glucan fraction with cellulase digests the glucan and also releases protein that has been characterized as GhCesA-1 and GhCesA-2--proteins that are believed to represent the catalytic subunit of cellulose synthase. The fact that cellulase treatment is required to release this protein indicates an extremely tight association of the glucan with the CesA proteins. In addition, CGA 325'615, but not DCB, also causes accumulation of CesA protein and a membrane-associated cellulase in the membrane fraction of fibers. In addition to the effects of CGA 325'615 on levels of both of these proteins, the level of both also shows coordinate regulation during fiber development, further suggesting they are both important for cellulose synthesis. The accumulation of non-crystalline glucan caused by CGA 325'615 mimics the phenotype of the cellulose-deficient rsw1 mutant of Arabidopsis that also accumulates an apparently similar glucan (T. Arioli, L. Peng, A.S. Betzner, J. Burn, W. Wittke, W. Herth, C. Camilleri, H. Hofte, J. Plazinski, R. Birch et al. [1998] Science 279: 717).
Figures





Similar articles
-
Sitosterol-beta-glucoside as primer for cellulose synthesis in plants.Science. 2002 Jan 4;295(5552):147-50. doi: 10.1126/science.1064281. Science. 2002. PMID: 11778054
-
Dimerization of cotton fiber cellulose synthase catalytic subunits occurs via oxidation of the zinc-binding domains.Proc Natl Acad Sci U S A. 2002 Aug 20;99(17):11109-14. doi: 10.1073/pnas.162077099. Epub 2002 Aug 1. Proc Natl Acad Sci U S A. 2002. PMID: 12154226 Free PMC article.
-
Molecular analysis of cellulose biosynthesis in Arabidopsis.Science. 1998 Jan 30;279(5351):717-20. doi: 10.1126/science.279.5351.717. Science. 1998. PMID: 9445479
-
Cellulose biosynthesis in plants: from genes to rosettes.Plant Cell Physiol. 2002 Dec;43(12):1407-20. doi: 10.1093/pcp/pcf164. Plant Cell Physiol. 2002. PMID: 12514238 Review.
-
Cellulose synthesis: a complex complex.Curr Opin Plant Biol. 2008 Jun;11(3):252-7. doi: 10.1016/j.pbi.2008.03.007. Epub 2008 May 14. Curr Opin Plant Biol. 2008. PMID: 18485800 Review.
Cited by
-
OsCESA9 conserved-site mutation leads to largely enhanced plant lodging resistance and biomass enzymatic saccharification by reducing cellulose DP and crystallinity in rice.Plant Biotechnol J. 2017 Sep;15(9):1093-1104. doi: 10.1111/pbi.12700. Epub 2017 Mar 15. Plant Biotechnol J. 2017. PMID: 28117552 Free PMC article.
-
Pausing of Golgi bodies on microtubules regulates secretion of cellulose synthase complexes in Arabidopsis.Plant Cell. 2009 Apr;21(4):1141-54. doi: 10.1105/tpc.108.065334. Epub 2009 Apr 17. Plant Cell. 2009. PMID: 19376932 Free PMC article.
-
Oxaziclomefone, a new herbicide, inhibits wall expansion in maize cell-cultures without affecting polysaccharide biosynthesis, xyloglucan transglycosylation, peroxidase action or apoplastic ascorbate oxidation.Ann Bot. 2005 Nov;96(6):1097-107. doi: 10.1093/aob/mci261. Epub 2005 Sep 6. Ann Bot. 2005. PMID: 16144873 Free PMC article.
-
Essential amino acids in the Plant-Conserved and Class-Specific Regions of cellulose synthases.Plant Physiol. 2023 Jan 2;191(1):142-160. doi: 10.1093/plphys/kiac479. Plant Physiol. 2023. PMID: 36250895 Free PMC article.
-
Resistance against herbicide isoxaben and cellulose deficiency caused by distinct mutations in same cellulose synthase isoform CESA6.Plant Physiol. 2002 Feb;128(2):482-90. doi: 10.1104/pp.010822. Plant Physiol. 2002. PMID: 11842152 Free PMC article.
References
-
- Arioli T, Peng L, Betzner AS, Burn J, Wittke W, Herth W, Camilleri C, Hofte H, Plazinski J, Birch R. Molecular analysis of cellulose biosynthesis in Arabidopsis. Science. 1998;279:717–720. - PubMed
-
- Blakeney AB, Harris PJ, Henry RJ, Stone BA. Simple and raid preparation of alditol acetates for monosaccharide analysis. Carbohydr Res. 1983;113:291–299.
-
- Carpita NC, Vergara C. A recipe for cellulose. Science. 1998;279:672–673. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

