The Ca(2+) status of the endoplasmic reticulum is altered by induction of calreticulin expression in transgenic plants
- PMID: 11457960
- PMCID: PMC116466
- DOI: 10.1104/pp.126.3.1092
The Ca(2+) status of the endoplasmic reticulum is altered by induction of calreticulin expression in transgenic plants
Abstract
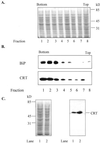
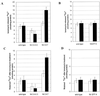
To investigate the endoplasmic reticulum (ER) Ca(2+) stores in plant cells, we generated tobacco (Nicotiana tabacum; NT1) suspension cells and Arabidopsis plants with altered levels of calreticulin (CRT), an ER-localized Ca(2+)-binding protein. NT1 cells and Arabidopsis plants were transformed with a maize (Zea mays) CRT gene in both sense and antisense orientations under the control of an Arabidopsis heat shock promoter. ER-enriched membrane fractions from NT1 cells were used to examine how altered expression of CRT affects Ca(2+) uptake and release. We found that a 2.5-fold increase in CRT led to a 2-fold increase in ATP-dependent (45)Ca(2+) accumulation in the ER-enriched fraction compared with heat-shocked wild-type controls. Furthermore, after treatment with the Ca(2+) ionophore ionomycin, ER microsomes from NT1 cells overproducing CRT showed a 2-fold increase in the amount of (45)Ca(2+) released, and a 2- to 3-fold increase in the amount of (45)Ca(2+) retained compared with wild type. These data indicate that altering the production of CRT affects the ER Ca(2+) pool. In addition, CRT transgenic Arabidopsis plants were used to determine if altered CRT levels had any physiological effects. We found that the level of CRT in heat shock-induced CRT transgenic plants correlated positively with the retention of chlorophyll when the plants were transferred from Ca(2+)-containing medium to Ca(2+)-depleted medium. Together these data are consistent with the hypothesis that increasing CRT in the ER increases the ER Ca(2+) stores and thereby enhances the survival of plants grown in low Ca(2+) medium.
Figures



Similar articles
-
Expression of the high capacity calcium-binding domain of calreticulin increases bioavailable calcium stores in plants.Transgenic Res. 2002 Feb;11(1):1-10. doi: 10.1023/a:1013917701701. Transgenic Res. 2002. PMID: 11874098
-
An ER-targeted calcium-binding peptide confers salt and drought tolerance mediated by CIPK6 in Arabidopsis.Planta. 2012 Mar;235(3):539-52. doi: 10.1007/s00425-011-1522-9. Epub 2011 Oct 5. Planta. 2012. PMID: 21971994
-
Ectopic expression of a maize calreticulin mitigates calcium deficiency-like disorders in sCAX1-expressing tobacco and tomato.Plant Mol Biol. 2012 Dec;80(6):609-19. doi: 10.1007/s11103-012-9970-6. Epub 2012 Sep 25. Plant Mol Biol. 2012. PMID: 23007728
-
Calreticulin: one protein, one gene, many functions.Biochem J. 1999 Dec 1;344 Pt 2(Pt 2):281-92. Biochem J. 1999. PMID: 10567207 Free PMC article. Review.
-
Calreticulin: non-endoplasmic reticulum functions in physiology and disease.FASEB J. 2010 Mar;24(3):665-83. doi: 10.1096/fj.09-145482. Epub 2009 Nov 25. FASEB J. 2010. PMID: 19940256 Free PMC article. Review.
Cited by
-
Transient dissociation of polyribosomes and concurrent recruitment of calreticulin and calmodulin transcripts in gravistimulated maize pulvini.Plant Physiol. 2001 Nov;127(3):1193-203. Plant Physiol. 2001. PMID: 11706198 Free PMC article.
-
Loss of all three calreticulins, CRT1, CRT2 and CRT3, causes enhanced sensitivity to water stress in Arabidopsis.Plant Cell Rep. 2013 Dec;32(12):1843-53. doi: 10.1007/s00299-013-1497-z. Plant Cell Rep. 2013. PMID: 24022063
-
Genome-wide identification, classification, and characterization of lectin gene superfamily in sweet orange (Citrus sinensis L.).PLoS One. 2023 Nov 13;18(11):e0294233. doi: 10.1371/journal.pone.0294233. eCollection 2023. PLoS One. 2023. PMID: 37956187 Free PMC article.
-
Diverging functions among calreticulin isoforms in higher plants.Plant Signal Behav. 2011 Jun;6(6):905-10. doi: 10.4161/psb.6.6.15339. Epub 2011 Jun 1. Plant Signal Behav. 2011. PMID: 21586899 Free PMC article.
-
HaCRT1 of Heterodera avenae Is Required for the Pathogenicity of the Cereal Cyst Nematode.Front Plant Sci. 2020 Nov 19;11:583584. doi: 10.3389/fpls.2020.583584. eCollection 2020. Front Plant Sci. 2020. PMID: 33329646 Free PMC article.
References
-
- Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF. Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science. 2000;289:2338–2342. - PubMed
-
- Allen GJ, Kwak JM, Chu SP, Llopis J, Tsien RY, Harper JF, Schroeder JI. Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J. 1999;19:735–747. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

