Inhibition of proteasome activity strongly affects kiwifruit pollen germination. Involvement of the ubiquitin/proteasome pathway as a major regulator
- PMID: 11457965
- PMCID: PMC116471
- DOI: 10.1104/pp.126.3.1150
Inhibition of proteasome activity strongly affects kiwifruit pollen germination. Involvement of the ubiquitin/proteasome pathway as a major regulator
Abstract
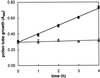
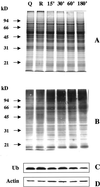
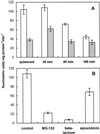
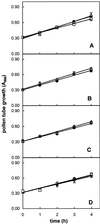
The 26S proteasome is a multicatalytic complex that acts as primary protease of the ubiquitin-mediated proteolytic pathway in eukaryotes. We provide here the first evidence that the proteasome plays a key role in regulating pollen tube growth. Immunoblotting experiments revealed the presence of high levels of free ubiquitin and ubiquitin conjugates in rehydrated and germinating pollen of kiwifruit [Actinidia deliciosa var. deliciosa (A. Chev) C. F. Liang et A. R. Ferguson]. Proteasome activity, assayed fluorometrically, accompanied the progression of germination. Specific inhibitors of proteasome function such as benzyloxycarbonyl-leucinyl-leucinyl-leucinal (MG-132), clasto-lactacystin beta-lactone, and epoxomicin significantly decreased tube growth or altered tube morphology. High-molecular mass, ubiquitinated proteins accumulated in MG-132- and beta-lactone-treated pollen, indicating that proteasome function was effectively impaired. The inhibitors were also able to decrease in vitro proteasome activity in pollen extracts. Because MG-132 can inhibit calpains, as well as the proteasome, trans-epoxy succinyl-L-leucylamido-(4-guanidino) butane (E-64), an inhibitor of cysteine proteases, was investigated. Some reduction in tube growth rate was observed, but only at 80 microM E-64, and no abnormal tubes were produced. Furthermore, no inhibition of tube growth was observed when another inhibitor of cysteine proteases, leupeptin, or inhibitors of serine and aspartic proteases (phenylmethylsulfonyl fluoride and pepstatin) were used. Our results indicate that protein turnover during tube organization and elongation in kiwifruit pollen is important, and our results also implicate the ubiquitin/26S proteasome as the major proteolytic pathway involved.
Figures


References
-
- Alché JD, Butowt R, Castro AJ, Rodriguez-Garcia MI. Ubiquitin and ubiquitin-conjugated proteins in the olive (Olea europea L.) pollen. Sex Plant Reprod. 2000;12:285–291.
-
- Bahrami AR, Gray JE. Conservation of proteasome structure and activity between plants and other eukaryotes. Biochem Soc Trans. 1998;26:395. - PubMed
-
- Becker F, Buschfeld E, Schell E, Bachmair A. Altered response to viral infection by tobacco plants perturbed in ubiquitin system. Plant J. 1993;3:875–881.
-
- Becker J, Kempf R, Jeblick W, Kauss H. Induction of competence for elicitation of defense responses in cucumber hypocotyls requires proteasome activity. Plant J. 2000;21:311–316. - PubMed
-
- Belknap WR, Garbarino JE. The role of ubiquitin in plant senescence and stress response. Trends Plant Sci. 1996;1:331–335.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

