Mn K-edge XANES and Kbeta XES studies of two Mn-oxo binuclear complexes: investigation of three different oxidation states relevant to the oxygen-evolving complex of photosystem II
- PMID: 11459481
- PMCID: PMC3959873
- DOI: 10.1021/ja004306h
Mn K-edge XANES and Kbeta XES studies of two Mn-oxo binuclear complexes: investigation of three different oxidation states relevant to the oxygen-evolving complex of photosystem II
Abstract
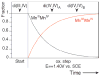
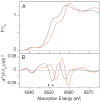
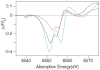
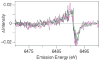
Two structurally homologous Mn compounds in different oxidation states were studied to investigate the relative influence of oxidation state and ligand environment on Mn K-edge X-ray absorption near-edge structure (XANES) and Mn Kbeta X-ray emission spectroscopy (Kbeta XES). The two manganese compounds are the di-mu-oxo compound [L'2Mn(III)O2Mn(IV)L'2](ClO4)3, where L' is 1,10-phenanthroline (Cooper, S. R.; Calvin, M. J. Am. Chem. Soc. 1977, 99, 6623-6630) and the linear mono-mu-oxo compound [LMn(III)OMn(III)L](ClO4)2, where L- is the monoanionic N,N-bis(2-pyridylmethyl)-N'-salicylidene-1,2-diaminoethane ligand (Horner, O.; Anxolabéhère-Mallart, E.; Charlot, M. F.; Tchertanov, L.; Guilhem, J.; Mattioli, T. A.; Boussac, A.; Girerd, J.-J. Inorg. Chem. 1999, 38, 1222-1232). Preparative bulk electrolysis in acetonitrile was used to obtain higher oxidation states of the compounds: the Mn(IV)Mn(IV) species for the di-mu-oxo compound and the Mn(III)Mn(IV) and Mn(IV)Mn(IV) species for the mono-mu-oxo compound. IR, UV/vis, EPR, and EXAFS spectra were used to determine the purity and integrity of the various sample solutions. The Mn K-edge XANES spectra shift to higher energy upon oxidation when the ligand environment remains similar. However, shifts in energy are also observed when only the ligand environment is altered. This is achieved by comparing the di-mu-oxo and linear mono-mu-oxo Mn-Mn moieties in equivalent oxidation states, which represent major structural changes. The magnitude of an energy shift due to major changes in ligand environment can be as large as that of an oxidation-state change. Therefore, care must be exercised when correlating the Mn K-edge energies to manganese oxidation states without taking into account the nature of the ligand environment and the overall structure of the compound. In contrast to Mn K-edge XANES, Kbeta XES spectra show less dependence on ligand environment. The Kbeta1,3 peak energies are comparable for the di-mu-oxo and mono-mu-oxo compounds in equivalent oxidation states. The energy shifts observed due to oxidation are also similar for the two different compounds. The study of the different behavior of the XANES pre-edge and main-edge features in conjunction with Kbeta XES provides significant information about the oxidation state and character of the ligand environment of manganese atoms.
Figures





References
-
- Cooper SR, Calvin M. J Am Chem Soc. 1977;99:6623–6630.
-
- Horner O, Anxolabéhère-Mallart E, Charlot MF, Tchertanov L, Guilhem J, Mattioli TA, Boussac A, Girerd J-J. Inorg Chem. 1999;38:1222–1232. - PubMed
-
- Kirby JA, Goodin DB, Wydrzynski T, Robertson AS, Klein MP. J Am Chem Soc. 1981;103:5537–5542.
-
- Yachandra VK, Sauer K, Klein MP. Chem Rev. 1996;96:2927–2950. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

