Pathological apoptosis by xanthurenic acid, a tryptophan metabolite: activation of cell caspases but not cytoskeleton breakdown
- PMID: 11459518
- PMCID: PMC34606
- DOI: 10.1186/1472-6793-1-7
Pathological apoptosis by xanthurenic acid, a tryptophan metabolite: activation of cell caspases but not cytoskeleton breakdown
Abstract
Background: A family of aspartate-specific cysteinyl proteases, named caspases, mediates programmed cell death, apoptosis. In this function, caspases are important for physiological processes such as development and maintenance of organ homeostasis. Caspases are, however, also engaged in aging and disease development. The factors inducing age-related caspase activation are not known. Xanthurenic acid, a product of tryptophan degradation, is present in blood and urine, and accumulates in organs with aging.
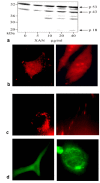
Results: Here, we report triggering of apoptotic key events by xanthurenic acid in vascular smooth muscle and retinal pigment epithelium cells. Upon exposure of these cells to xanthurenic acid a degradation of ICAD/DFF45, poly(ADP-ribose) polymerase, and gelsolin was observed, giving a pattern of protein cleavage characteristic for caspase-3 activity. Active caspase-3, -8 and caspase-9 were detected by Western blot analysis and immunofluorescence. In the presence of xanthurenic acid the amino-terminal fragment of gelsolin bound to the cytoskeleton, but did not lead to the usually observed cytoskeleton breakdown. Xanthurenic acid also caused mitochondrial migration, cytochrome C release, and destruction of mitochondria and nuclei.
Conclusions: These results indicate that xanthurenic acid is a previously not recognized endogenous cell death factor. Its accumulation in cells may lead to accelerated caspase activation related to aging and disease development.
Figures



Similar articles
-
Lens epithelial cell apoptosis and intracellular Ca2+ increase in the presence of xanthurenic acid.BMC Ophthalmol. 2002 Apr 5;2:1. doi: 10.1186/1471-2415-2-1. BMC Ophthalmol. 2002. PMID: 11934353 Free PMC article.
-
Xanthurenic acid translocates proapoptotic Bcl-2 family proteins into mitochondria and impairs mitochondrial function.BMC Cell Biol. 2004 Apr 6;5:14. doi: 10.1186/1471-2121-5-14. BMC Cell Biol. 2004. PMID: 15068490 Free PMC article.
-
Cleavage of DFF-45/ICAD by multiple caspases is essential for its function during apoptosis.J Biol Chem. 1998 Oct 30;273(44):28549-52. doi: 10.1074/jbc.273.44.28549. J Biol Chem. 1998. PMID: 9786842
-
Caspase-3-induced gelsolin fragmentation contributes to actin cytoskeletal collapse, nucleolysis, and apoptosis of vascular smooth muscle cells exposed to proinflammatory cytokines.Eur J Cell Biol. 1998 Dec;77(4):294-302. doi: 10.1016/S0171-9335(98)80088-5. Eur J Cell Biol. 1998. PMID: 9930654
-
Making the head: Caspases in life and death.Front Cell Dev Biol. 2023 Jan 13;10:1075751. doi: 10.3389/fcell.2022.1075751. eCollection 2022. Front Cell Dev Biol. 2023. PMID: 36712975 Free PMC article. Review.
Cited by
-
Effects of Various Kynurenine Metabolites on Respiratory Parameters of Rat Brain, Liver and Heart Mitochondria.Int J Tryptophan Res. 2016 May 17;9:17-29. doi: 10.4137/IJTR.S37973. eCollection 2016. Int J Tryptophan Res. 2016. PMID: 27226722 Free PMC article.
-
Protective actions of ovarian hormones in the serotonin system of macaques.Front Neuroendocrinol. 2009 Jul;30(2):212-38. doi: 10.1016/j.yfrne.2009.04.003. Epub 2009 Apr 24. Front Neuroendocrinol. 2009. PMID: 19394356 Free PMC article. Review.
-
Insulin resistance and dysregulation of tryptophan-kynurenine and kynurenine-nicotinamide adenine dinucleotide metabolic pathways.Mol Neurobiol. 2013 Oct;48(2):294-301. doi: 10.1007/s12035-013-8497-4. Epub 2013 Jun 28. Mol Neurobiol. 2013. PMID: 23813101 Free PMC article. Review.
-
Variable Dose Rates in Realistic Radiation Exposures: Effects on Small Molecule Markers of Ionizing Radiation in the Murine Model.Radiat Res. 2023 Jul 1;200(1):1-12. doi: 10.1667/RADE-22-00211.1. Radiat Res. 2023. PMID: 37212727 Free PMC article.
-
Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation.Front Biosci (Landmark Ed). 2015 Jun 1;20(7):1116-43. doi: 10.2741/4363. Front Biosci (Landmark Ed). 2015. PMID: 25961549 Free PMC article. Review.
References
-
- Carlin JM, Ozaki Y, Byrne GI, Brown RR, Borden EC. Interferons and indoleamine 2,3-dioxygenase: role in antimicrobial and antitumor effects. Experientia. 1989;45:535–541. - PubMed
-
- Tobes MC. Kynurenine-oxoglutarate aminotransferase from rat kidney. Methods Enzymol. 1987;142:217–224. - PubMed
-
- Mosca M, Cozzi L, Breton J, Avanzi N, Toma S, Okuno E, et al. Cloning of rat and human kynurenine aminotransferase. Adv Exp Med Biol. 1996;398:449–454. - PubMed
-
- Williams SA, Monti JA, Boots LR, Cornwell PE. Quantitation of xanthurenic acid in rabbit serum using high performance liquid chromatography. Am J Clin Nutr. 1984;40:159–167. - PubMed
-
- Cavill IA. Estimation of the tryptophan metabolites xanthurenic acid, 3-hydroxykynurenine and kynurenine. Clin Chim Acta. 1967;18:285–289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

