Carbohydrate self-recognition mediates marine sponge cellular adhesion
- PMID: 11459930
- PMCID: PMC55436
- DOI: 10.1073/pnas.151111298
Carbohydrate self-recognition mediates marine sponge cellular adhesion
Abstract
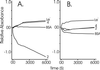
Sponges (Porifera), the simplest and earliest multicellular organisms, are thought to have evolved from their unicellular ancestors about 1 billion years ago by developing cell-recognition and adhesion mechanisms to discriminate against "non-self." Consequently, they are used as models for investigating recognition phenomena. Cellular adhesion of marine sponges is an event involving adherence of extracellular proteoglycan-like molecules, otherwise known as aggregation factors (AFs). In a calcium-independent process the AFs adhere to the cell surface, and in a calcium-dependent process they exhibit AF self-association. A mechanism which has been implied but not definitely proven to play a role in the calcium-dependent event is self-recognition of defined carbohydrate epitopes. For the red beard sponge, Microciona prolifera, two carbohydrate epitopes, a sulfated disaccharide and a pyruvylated trisaccharide, have been implicated in cellular adhesion. To investigate this phenomenon a system has been designed, by using surface plasmon resonance detection, to mimic the role of carbohydrates in cellular adhesion of M. prolifera. The results show self-recognition of the sulfated disaccharide to be a major force behind the calcium-dependent event. The interaction is not simply based on electrostatic interactions, as other sulfated carbohydrates analyzed by using this procedure did not self-associate. Furthermore, the interaction is completely eradicated on substitution of Ca(2+) ions by either Mg(2+) or Mn(2+) ions. This physiologically relevant recognition mechanism confirms the existence of true carbohydrate self-recognition, and may have significant implications for the role of carbohydrates in cellular recognition of higher organisms.
Figures
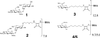






References
-
- Eggens I, Fenderson B A, Toyokuni T, Dean B, Stroud M R, Hakomori S-I. J Biol Chem. 1989;264:9976–9984. - PubMed
-
- Kojima N, Fenderson B A, Stroud M R, Goldberg R I, Habermann R, Toyokuni T, Hakomori S-I. Glycoconj J. 1994;11:238–248. - PubMed
-
- Hakomori S-I. Pure Appl Chem. 1991;63:473–482.
-
- Bovin N V. In: Glycosciences. Gabius H-J, Gabius S, editors. Weinheim, Germany: Chapman and Hall; 1997. pp. 277–289.
-
- Geyer A, Gege C, Schmidt R R. Angew Chem Int Ed Engl. 1999;38:1466–1468. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

