Angiogenic role for glycodelin in tumorigenesis
- PMID: 11459932
- PMCID: PMC55409
- DOI: 10.1073/pnas.151151198
Angiogenic role for glycodelin in tumorigenesis
Abstract
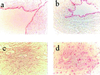
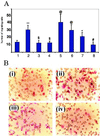
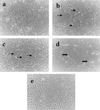
Angiogenesis plays an important role in neovascularization in tumors. Glycodelin, a hormone-responsive protein, has been detected in tumors of reproductive organs and is found in high levels in the plasma of subjects with gynecological malignancies. Glycodelin is also found in the endothelial cells of the umbilical cord and in the blood vessels of tumors. In this study, we tested whether glycodelin-rich amniotic fluid and a synthetic peptide derived from the sequence of glycodelin peptide (Gp) might promote angiogenic response by examining the migration and tube formation in human umbilical cord vein endothelial cells (HUVECs). Increased migration and tube formation of HUVECs were found in the presence of amniotic fluid and Gp, and this increase was blocked by antibody to Gp and by an anti-vascular endothelial growth factor (VEGF) antibody, suggesting that the angiogenic effects of glycodelin might be mediated by VEGF. The results also showed that Gp significantly increased the release of VEGF protein and mRNA expression in HUVECs, RL-95 (human endometrial carcinoma cells), OVCAR-3 (human ovarian adenocarcinoma cells), EM42 (human endometrial epithelial cells), THP-1 (human monocyte), and MCF-7 and MDA-MB-231 (human breast adenocarcinoma cells) cell lines. VEGF receptor Fit-1 mRNA expression in HUVECs was also increased in the presence of Gp. These findings, together with the suggestion from the literature that glycodelin may have immunosuppressive properties, suggest that glycodelin might play an important role in neovascularization during embryogenesis and tumor development.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

