Holins kill without warning
- PMID: 11459934
- PMCID: PMC55423
- DOI: 10.1073/pnas.151247598
Holins kill without warning
Abstract
Holins comprise the most diverse functional group of proteins known. They are small bacteriophage-encoded proteins that accumulate during the period of late-protein synthesis after infection and cause lysis of the host cell at a precise genetically programmed time. It is unknown how holins achieve temporal precision, but a conserved feature of their function is that energy poisons subvert the normal scheduling mechanism and instantly trigger membrane disruption. On this basis, timing has been proposed to involve a progressive decrease in the energized state of the membrane until a critical triggering level is reached. Here, we report that membrane integrity is not compromised after the induction of holin synthesis until seconds before lysis. The proton motive force was monitored by the rotation of individual cells tethered by a single flagellum. The results suggest an alternative explanation for the lysis "clock," in which holin concentrations build to a critical level that leads to formation of an oligomeric complex that disrupts the membrane.
Figures
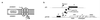

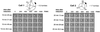

References
-
- Wang I-N, Smith D L, Young R. Annu Rev Microbiol. 2000;54:799–825. - PubMed
-
- Young R, Wang I-N, Roof W D. Trends Microbiol. 2000;8:120–128. - PubMed
-
- Wang I-N, Dykhuizen D E, Slobodkin L B. Evol Ecol. 1996;10:545–558.
-
- Raab R, Neal G, Sohaskey C, Smith J, Young R. J Mol Biol. 1988;199:95–105. - PubMed
-
- Johnson-Boaz R, Chang C-Y, Young R. Mol Microbiol. 1994;13:495–504. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

