A yeast gene, MGS1, encoding a DNA-dependent AAA(+) ATPase is required to maintain genome stability
- PMID: 11459965
- PMCID: PMC37433
- DOI: 10.1073/pnas.121009098
A yeast gene, MGS1, encoding a DNA-dependent AAA(+) ATPase is required to maintain genome stability
Abstract
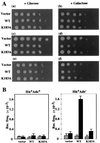
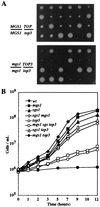
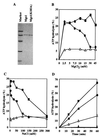
Changes in DNA superhelicity during DNA replication are mediated primarily by the activities of DNA helicases and topoisomerases. If these activities are defective, the progression of the replication fork can be hindered or blocked, which can lead to double-strand breaks, elevated recombination in regions of repeated DNA, and genome instability. Hereditary diseases like Werner's and Bloom's Syndromes are caused by defects in DNA helicases, and these diseases are associated with genome instability and carcinogenesis in humans. Here we report a Saccharomyces cerevisiae gene, MGS1 (Maintenance of Genome Stability 1), which encodes a protein belonging to the AAA(+) class of ATPases, and whose central region is similar to Escherichia coli RuvB, a Holliday junction branch migration motor protein. The Mgs1 orthologues are highly conserved in prokaryotes and eukaryotes. The Mgs1 protein possesses DNA-dependent ATPase and single-strand DNA annealing activities. An mgs1 deletion mutant has an elevated rate of mitotic recombination, which causes genome instability. The mgs1 mutation is synergistic with a mutation in top3 (encoding topoisomerase III), and the double mutant exhibits severe growth defects and markedly increased genome instability. In contrast to the mgs1 mutation, a mutation in the sgs1 gene encoding a DNA helicase homologous to the Werner and Bloom helicases suppresses both the growth defect and the increased genome instability of the top3 mutant. Therefore, evolutionarily conserved Mgs1 may play a role together with RecQ family helicases and DNA topoisomerases in maintaining proper DNA topology, which is essential for genome stability.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

