Instability of repetitive DNA sequences: the role of replication in multiple mechanisms
- PMID: 11459970
- PMCID: PMC37438
- DOI: 10.1073/pnas.111008398
Instability of repetitive DNA sequences: the role of replication in multiple mechanisms
Abstract
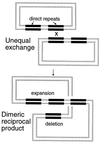
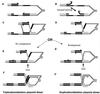
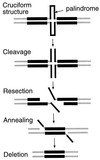
Rearrangements between tandem sequence homologies of various lengths are a major source of genomic change and can be deleterious to the organism. These rearrangements can result in either deletion or duplication of genetic material flanked by direct sequence repeats. Molecular genetic analysis of repetitive sequence instability in Escherichia coli has provided several clues to the underlying mechanisms of these rearrangements. We present evidence for three mechanisms of RecA-independent sequence rearrangements: simple replication slippage, sister-chromosome exchange-associated slippage, and single-strand annealing. We discuss the constraints of these mechanisms and contrast their properties with RecA-dependent homologous recombination. Replication plays a critical role in the two slipped misalignment mechanisms, and difficulties in replication appear to trigger rearrangements via all these mechanisms.
Figures


References
-
- Morag A S, Saveson C J, Lovett S T. J Mol Biol. 1999;289:21–27. - PubMed
-
- Kowalczykowski S C. Trends Biochem Sci. 2000;25:156–165. - PubMed
-
- Farabaugh P J, Schmeissner U, Hofer M, Miller J H. J Mol Biol. 1978;126:847–857. - PubMed
-
- Krawczak M, Cooper D N. Hum Genet. 1991;86:425–441. - PubMed
-
- Meuth M. In: Mobile DNA. Berg D E, Howe M M, editors. Washington, DC: Am. Soc. Microbiol.; 1989. pp. 833–860.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

