Two overlapping subdominant epitopes identified by DNA immunization induce protective CD8(+) T-cell populations with differing cytolytic activities
- PMID: 11462012
- PMCID: PMC114975
- DOI: 10.1128/JVI.75.16.7399-7409.2001
Two overlapping subdominant epitopes identified by DNA immunization induce protective CD8(+) T-cell populations with differing cytolytic activities
Abstract
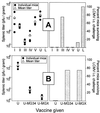
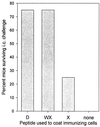
Subdominant CD8(+) T-cell responses contribute to control of several viral infections and to vaccine-induced immunity. Here, using the lymphocytic choriomeningitis virus model, we demonstrate that subdominant epitopes can be more reliably identified by DNA immunization than by other methods, permitting the identification, in the virus nucleoprotein, of two overlapping subdominant epitopes: one presented by L(d) and the other presented by K(d). This subdominant sequence confers immunity as effective as that induced by the dominant epitope, against which >90% of the antiviral CD8(+) T cells are normally directed. We compare the kinetics of the dominant and subdominant responses after vaccination with those following subsequent viral infection. The dominant CD8(+) response expands more rapidly than the subdominant responses, but after virus infection is cleared, mice which had been immunized with the "dominant" vaccine have a pool of memory T cells focused almost entirely upon the dominant epitope. In contrast, after virus infection, mice which had been immunized with the "subdominant" vaccine retain both dominant and subdominant memory cells. During the acute phase of the immune response, the acquisition of cytokine responsiveness by subdominant CD8(+) T cells precedes their development of lytic activity. Furthermore, in both dominant and subdominant populations, lytic activity declines more rapidly than cytokine responsiveness. Thus, the lysis(low)-cytokine(competent) phenotype associated with most memory CD8(+) T cells appears to develop soon after antigen clearance. Finally, lytic activity differs among CD8(+) T-cell populations with different epitope specificities, suggesting that vaccines can be designed to selectively induce CD8(+) T cells with distinct functional attributes.
Figures






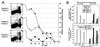
Similar articles
-
Immunodominance in virus-induced CD8(+) T-cell responses is dramatically modified by DNA immunization and is regulated by gamma interferon.J Virol. 2002 May;76(9):4251-9. doi: 10.1128/jvi.76.9.4251-4259.2002. J Virol. 2002. PMID: 11932390 Free PMC article.
-
Efficient priming of CD8+ memory T cells specific for a subdominant epitope following Sendai virus infection.J Immunol. 1997 May 1;158(9):4301-9. J Immunol. 1997. PMID: 9126992
-
Inefficient cross-presentation limits the CD8+ T cell response to a subdominant tumor antigen epitope.J Immunol. 2005 Jul 15;175(2):700-12. doi: 10.4049/jimmunol.175.2.700. J Immunol. 2005. PMID: 16002665
-
Vaccine Targeting of Subdominant CD8+ T Cell Epitopes Increases the Breadth of the T Cell Response upon Viral Challenge, but May Impair Immediate Virus Control.J Immunol. 2016 Mar 15;196(6):2666-76. doi: 10.4049/jimmunol.1502018. Epub 2016 Feb 12. J Immunol. 2016. PMID: 26873995
-
Analysis of cytotoxic T cell responses to dominant and subdominant epitopes during acute and chronic lymphocytic choriomeningitis virus infection.J Immunol. 1996 Dec 15;157(12):5543-54. J Immunol. 1996. PMID: 8955205
Cited by
-
Strong CD8 T-cell responses following coimmunization with plasmids expressing the dominant pp89 and subdominant M84 antigens of murine cytomegalovirus correlate with long-term protection against subsequent viral challenge.J Virol. 2002 Mar;76(5):2100-12. doi: 10.1128/jvi.76.5.2100-2112.2002. J Virol. 2002. PMID: 11836387 Free PMC article.
-
TCR affinity associated with functional differences between dominant and subdominant SIV epitope-specific CD8+ T cells in Mamu-A*01+ rhesus monkeys.PLoS Pathog. 2014 Apr 17;10(4):e1004069. doi: 10.1371/journal.ppat.1004069. eCollection 2014 Apr. PLoS Pathog. 2014. PMID: 24743648 Free PMC article.
-
Characterization of CD8+ T cell function and immunodominance generated with an H2O2-inactivated whole-virus vaccine.J Virol. 2012 Dec;86(24):13735-44. doi: 10.1128/JVI.02178-12. Epub 2012 Oct 10. J Virol. 2012. PMID: 23055558 Free PMC article.
-
Two antigenic peptides from genes m123 and m164 of murine cytomegalovirus quantitatively dominate CD8 T-cell memory in the H-2d haplotype.J Virol. 2002 Jan;76(1):151-64. doi: 10.1128/jvi.76.1.151-164.2002. J Virol. 2002. PMID: 11739681 Free PMC article.
-
MHC basis of T cell-dependent heterologous immunity to arenaviruses.Virology. 2014 Sep;464-465:213-217. doi: 10.1016/j.virol.2014.07.012. Epub 2014 Aug 3. Virology. 2014. PMID: 25094042 Free PMC article.
References
-
- An L L, Rodriguez F, Harkins S, Zhang J, Whitton J L. Quantitative and qualitative analyses of the immune responses induced by a multivalent minigene DNA vaccine. Vaccine. 2000;18:2132–2141. - PubMed
-
- Bachmann M F, Barner M, Viola A, Kopf M. Distinct kinetics of cytokine production and cytolysis in effector and memory T cells after viral infection. Eur J Immunol. 1999;29:291–299. - PubMed
-
- Borrow P, Lewicki H, Wei X, Horwitz M S, Peffer N, Meyers H, Nelson J A, Gairin J E, Hahn B H, Oldstone M B A, Shaw G M. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. - PubMed
-
- Chen Y, Webster R G, Woodland D L. Induction of CD8+ T cell responses to dominant and subdominant epitopes and protective immunity to Sendai virus infection by DNA vaccination. J Immunol. 1998;160:2425–2432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

