High numbers of viral RNA copies in the central nervous system of mice during persistent infection with Theiler's virus
- PMID: 11462014
- PMCID: PMC114977
- DOI: 10.1128/JVI.75.16.7420-7428.2001
High numbers of viral RNA copies in the central nervous system of mice during persistent infection with Theiler's virus
Abstract
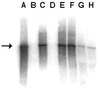
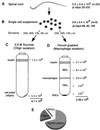
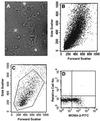
The low-neurovirulence Theiler's murine encephalomyelitis viruses (TMEV), such as BeAn virus, cause a persistent infection of the central nervous system (CNS) in susceptible mouse strains that results in inflammatory demyelination. The ability of TMEV to persist in the mouse CNS has traditionally been demonstrated by recovering infectious virus from the spinal cord. Results of infectivity assays led to the notion that TMEV persists at low levels. In the present study, we analyzed the copy number of TMEV genomes, plus- to minus-strand ratios, and full-length species in the spinal cords of infected mice and infected tissue culture cells by using Northern hybridization. Considering the low levels of infectious virus in the spinal cord, a surprisingly large number of viral genomes (mean of 3.0 x 10(9)) was detected in persistently infected mice. In the transition from the acute (approximately postinfection [p.i.] day 7) to the persistent (beginning on p.i. day 28) phase of infection, viral RNA copy numbers steadily increased, indicating that TMEV persistence involves active viral RNA replication. Further, BeAn viral genomes were full-length in size; i.e., no subgenomic species were detected and the ratio of BeAn virus plus- to minus-strand RNA indicated that viral RNA replication is unperturbed in the mouse spinal cord. Analysis of cultured macrophages and oligodendrocytes suggests that either of these cell types can potentially synthesize high numbers of viral RNA copies if infected in the spinal cord and therefore account for the heavy viral load. A scheme is presented for the direct isolation of both cell types directly from infected spinal cords for further viral analyses.
Figures


References
-
- Aubert C, Chamorro M, Brahic M. Identification of Theiler's virus infected cells in the central nervous system of the mouse during demyelinating disease. Microb Pathog. 1987;3:319–326. - PubMed
-
- Blakemore W F, Welsh C J, Tonks P, Nash A A. Observations on demyelinating lesions induced by Theiler's virus in CBA mice. Acta Neuropathol. 1988;76:581–589. - PubMed
-
- Cash E, Brahic M. Quantitative in situ hybridization using initial velocity measurements. Anal Biochem. 1986;157:266–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

