Protection against simian immunodeficiency virus vaginal challenge by using Sabin poliovirus vectors
- PMID: 11462016
- PMCID: PMC114979
- DOI: 10.1128/JVI.75.16.7435-7452.2001
Protection against simian immunodeficiency virus vaginal challenge by using Sabin poliovirus vectors
Abstract
Here we provide the first report of protection against a vaginal challenge with a highly virulent simian immunodeficiency virus (SIV) by using a vaccine vector. New poliovirus vectors based on Sabin 1 and 2 vaccine strain viruses were constructed, and these vectors were used to generate a series of new viruses containing SIV gag, pol, env, nef, and tat in overlapping fragments. Two cocktails of 20 transgenic polioviruses (SabRV1-SIV and SabRV2-SIV) were inoculated into seven cynomolgus macaques. All monkeys produced substantial anti-SIV serum and mucosal antibody responses. SIV-specific cytotoxic T-lymphocyte responses were detected in three of seven monkeys after vaccination. All 7 vaccinated macaques, as well as 12 control macaques, were challenged vaginally with pathogenic SIVmac251. Strikingly, four of the seven vaccinated animals exhibited substantial protection against the vaginal SIV challenge. All 12 control monkeys became SIV positive. In two of the seven SabRV-SIV-vaccinated monkeys we found no virological evidence of infection following challenge, indicating that these two monkeys were completely protected. Two additional SabRV-SIV-vaccinated monkeys exhibited a pronounced reduction in postacute viremia to <10(3) copies/ml, suggesting that the vaccine elicited an effective cellular immune response. Three of six control animals developed clinical AIDS by 48 weeks postchallenge. In contrast, all seven vaccinated monkeys remained healthy as judged by all clinical parameters. These results demonstrate the efficacy of SabRV as a potential human vaccine vector, and they show that the use of a vaccine vector cocktail expressing an array of defined antigenic sequences can be an effective vaccination strategy in an outbred population.
Figures
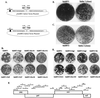




 ; 26405, ⋄;
26560, ▿; and 28118, ◂.
; 26405, ⋄;
26560, ▿; and 28118, ◂.
 ; 26405, ⋄; 26560, ▿; and 28118, ◂. Vaccinated monkeys
27270 and 27244 were never positive for SIV RNA and appeared to be
completely protected. The threshold sensitivity of the assay (indicated
by a dashed line) is 100 RNA copy equivalents/ml. Data points below the
threshold value are shown at 100. †, animal died between weeks 32 and
44 postchallenge.
; 26405, ⋄; 26560, ▿; and 28118, ◂. Vaccinated monkeys
27270 and 27244 were never positive for SIV RNA and appeared to be
completely protected. The threshold sensitivity of the assay (indicated
by a dashed line) is 100 RNA copy equivalents/ml. Data points below the
threshold value are shown at 100. †, animal died between weeks 32 and
44 postchallenge.
Similar articles
-
Protective efficacy of a multicomponent vector vaccine in cynomolgus monkeys after intrarectal simian immunodeficiency virus challenge.J Gen Virol. 2004 May;85(Pt 5):1191-1201. doi: 10.1099/vir.0.79794-0. J Gen Virol. 2004. PMID: 15105535
-
Vectored Gag and Env but not Tat show efficacy against simian-human immunodeficiency virus 89.6P challenge in Mamu-A*01-negative rhesus monkeys.J Virol. 2005 Oct;79(19):12321-31. doi: 10.1128/JVI.79.19.12321-12331.2005. J Virol. 2005. PMID: 16160159 Free PMC article.
-
Mucosal immunization of cynomolgus macaques with two serotypes of live poliovirus vectors expressing simian immunodeficiency virus antigens: stimulation of humoral, mucosal, and cellular immunity.J Virol. 1999 Nov;73(11):9485-95. doi: 10.1128/JVI.73.11.9485-9495.1999. J Virol. 1999. PMID: 10516057 Free PMC article.
-
Poliovirus vaccine strains as mucosal vaccine vectors and their potential use to develop an AIDS vaccine.Adv Drug Deliv Rev. 2004 Apr 19;56(6):835-52. doi: 10.1016/j.addr.2003.10.042. Adv Drug Deliv Rev. 2004. PMID: 15063593 Review.
-
Simian and feline immunodeficiency viruses: animal lentivirus models for evaluation of AIDS vaccines and antiviral agents.Antiviral Res. 1991 May;15(4):267-86. doi: 10.1016/0166-3542(91)90009-g. Antiviral Res. 1991. PMID: 1659310 Review.
Cited by
-
Protection against mucosal simian immunodeficiency virus SIV(mac251) challenge by using replicating adenovirus-SIV multigene vaccine priming and subunit boosting.J Virol. 2004 Mar;78(5):2212-21. doi: 10.1128/jvi.78.5.2212-2221.2004. J Virol. 2004. PMID: 14963117 Free PMC article.
-
Repeated low-dose mucosal simian immunodeficiency virus SIVmac239 challenge results in the same viral and immunological kinetics as high-dose challenge: a model for the evaluation of vaccine efficacy in nonhuman primates.J Virol. 2004 Mar;78(6):3140-4. doi: 10.1128/jvi.78.6.3140-3144.2004. J Virol. 2004. PMID: 14990733 Free PMC article.
-
A molecularly cloned Schwarz strain of measles virus vaccine induces strong immune responses in macaques and transgenic mice.J Virol. 2003 Nov;77(21):11546-54. doi: 10.1128/jvi.77.21.11546-11554.2003. J Virol. 2003. PMID: 14557640 Free PMC article.
-
Potent, persistent induction and modulation of cellular immune responses in rhesus macaques primed with Ad5hr-simian immunodeficiency virus (SIV) env/rev, gag, and/or nef vaccines and boosted with SIV gp120.J Virol. 2003 Aug;77(16):8607-20. doi: 10.1128/jvi.77.16.8607-8620.2003. J Virol. 2003. PMID: 12885879 Free PMC article.
-
A single injection of recombinant measles virus vaccines expressing human immunodeficiency virus (HIV) type 1 clade B envelope glycoproteins induces neutralizing antibodies and cellular immune responses to HIV.J Virol. 2004 Jan;78(1):146-57. doi: 10.1128/jvi.78.1.146-157.2004. J Virol. 2004. PMID: 14671096 Free PMC article.
References
-
- Abbas A K, Lichtman A H, Pober J S. Cellular and molecular immunology. 4th ed. Philadelphia, Pa: W. B. Saunders; 2000.
-
- AFHS. AFHS drug information. Bethesda, Md: SilverPlatter International; 1998.
-
- Ahmad S, Lohman B, Marthas M, Giavedoni L, el-Amad Z, Haigwood N L, Scandella C J, Gardner M B, Luciw P A, Yilma T. Reduced virus load in rhesus macaques immunized with recombinant gp160 and challenged with simian immunodeficiency virus. AIDS Res Hum Retrovir. 1994;10:195–204. - PubMed
-
- Andino R, Silvera D, Suggett S D, Achacoso P L, Miller C J, Baltimore D, Feinberg M B. Engineering poliovirus as a vaccine vector for the expression of diverse antigens. Science. 1994;265:1448–1451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

