Postnatal passive immunization of neonatal macaques with a triple combination of human monoclonal antibodies against oral simian-human immunodeficiency virus challenge
- PMID: 11462019
- PMCID: PMC114982
- DOI: 10.1128/JVI.75.16.7470-7480.2001
Postnatal passive immunization of neonatal macaques with a triple combination of human monoclonal antibodies against oral simian-human immunodeficiency virus challenge
Abstract
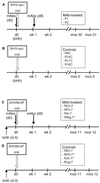
To develop prophylaxis against mother-to-child human immunodeficiency virus (HIV) transmission, we established a simian-human immunodeficiency virus (SHIV) infection model in neonatal macaques that mimics intrapartum mucosal virus exposure (T. W. Baba et al., AIDS Res. Hum. Retroviruses 10:351-357, 1994). Using this model, neonates were protected from mucosal SHIV-vpu(+) challenge by pre- and postnatal treatment with a combination of three human neutralizing monoclonal antibodies (MAbs), F105, 2G12, and 2F5 (Baba et al., Nat. Med. 6:200-206, 2000). In the present study, we used this MAb combination only postnatally, thereby significantly reducing the quantity of antibodies necessary and rendering their potential use in humans more practical. We protected two neonates with this regimen against oral SHIV-vpu(+) challenge, while four untreated control animals became persistently infected. Thus, synergistic MAbs protect when used as immunoprophylaxis without the prenatal dose. We then determined in vitro the optimal MAb combination against the more pathogenic SHIV89.6P, a chimeric virus encoding env of the primary HIV89.6. Remarkably, the most potent combination included IgG1b12, which alone does not neutralize SHIV89.6P. We administered the combination of MAbs IgG1b12, 2F5, and 2G12 postnatally to four neonates. One of the four infants remained uninfected after oral challenge with SHIV89.6P, and two infants had no or a delayed CD4(+) T-cell decline. In contrast, all control animals had dramatic drops in their CD4(+) T cells by 2 weeks postexposure. We conclude that our triple MAb combination partially protected against mucosal challenge with the highly pathogenic SHIV89.6P. Thus, combination immunoprophylaxis with passively administered synergistic human MAbs may play a role in the clinical prevention of mother-to-infant transmission of HIV type 1.
Figures
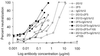


Similar articles
-
Protection of neonatal macaques against experimental SHIV infection by human neutralizing monoclonal antibodies.Transfus Clin Biol. 2001 Aug;8(4):350-8. doi: 10.1016/s1246-7820(01)00187-2. Transfus Clin Biol. 2001. PMID: 11642027 Review.
-
Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection.Nat Med. 2000 Feb;6(2):200-6. doi: 10.1038/72309. Nat Med. 2000. PMID: 10655110
-
Postnatal pre- and postexposure passive immunization strategies: protection of neonatal macaques against oral simian-human immunodeficiency virus challenge.J Med Primatol. 2002 Jun;31(3):109-19. doi: 10.1034/j.1600-0684.2002.01014.x. J Med Primatol. 2002. PMID: 12190851
-
Passive immunization against oral AIDS virus transmission: an approach to prevent mother-to-infant HIV-1 transmission?J Med Primatol. 2001 Aug;30(4):190-6. doi: 10.1034/j.1600-0684.2001.d01-52.x. J Med Primatol. 2001. PMID: 11555137
-
Antibody protection: passive immunization of neonates against oral AIDS virus challenge.Vaccine. 2003 Jul 28;21(24):3370-3. doi: 10.1016/s0264-410x(03)00335-9. Vaccine. 2003. PMID: 12850342 Review.
Cited by
-
Coverage of primary mother-to-child HIV transmission isolates by second-generation broadly neutralizing antibodies.AIDS. 2013 Jan 28;27(3):337-46. doi: 10.1097/QAD.0b013e32835cadd6. AIDS. 2013. PMID: 23296195 Free PMC article.
-
Neutralizing antibody escape during HIV-1 mother-to-child transmission involves conformational masking of distal epitopes in envelope.J Virol. 2012 Sep;86(18):9566-82. doi: 10.1128/JVI.00953-12. Epub 2012 Jun 27. J Virol. 2012. PMID: 22740394 Free PMC article.
-
Characterization of human class-switched polymeric (immunoglobulin M [IgM] and IgA) anti-human immunodeficiency virus type 1 antibodies 2F5 and 2G12.J Virol. 2003 Apr;77(7):4095-103. doi: 10.1128/jvi.77.7.4095-4103.2003. J Virol. 2003. PMID: 12634368 Free PMC article.
-
Immunoprophylaxis against mother-to-child transmission of HIV-1.PLoS Med. 2006 Jul;3(7):e259. doi: 10.1371/journal.pmed.0030259. Epub 2006 Jul 18. PLoS Med. 2006. PMID: 16842019 Free PMC article.
-
Antibody responses elicited in macaques immunized with human immunodeficiency virus type 1 (HIV-1) SF162-derived gp140 envelope immunogens: comparison with those elicited during homologous simian/human immunodeficiency virus SHIVSF162P4 and heterologous HIV-1 infection.J Virol. 2006 Sep;80(17):8745-62. doi: 10.1128/JVI.00956-06. J Virol. 2006. PMID: 16912322 Free PMC article.
References
-
- Anonymous. Intravenous immune globulin for the prevention of bacterial infections in children with symptomatic human immunodeficiency virus infection. The National Institute of Child Health and Human Developments Intravenous Immunoglobulin Study Group. N Engl J Med. 1991;325:73–80. - PubMed
-
- Baba T W, Koch J, Mittler E S, Greene M, Wyand M, Pennick D, Ruprecht R M. Mucosal infection of neonatal rhesus monkeys with cell-free SIV. AIDS Res Hum Retroviruses. 1994;10:351–357. - PubMed
-
- Baba T W, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini L A, Posner M R, Katinger H, Stiegler G, Bernacky B J, Rizvi T A, Schmidt R, Hill L R, Keeling M E, Lu Y, Wright J E, Chou T C, Ruprecht R M. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. - PubMed
-
- Barouch D H, Santra S, Schmitz J E, Kuroda M J, Fu T M, Wagner W, Bilska M, Craiu A, Zheng X X, Krivulka G R, Beaudry K, Lifton M A, Nickerson C E, Trigona W L, Punt K, Freed D C, Guan L, Dubey S, Casimiro D, Simon A, Davies M E, Chastain M, Strom T B, Gelman R S, Montefiori D C, Lewis M G. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–492. - PubMed
-
- Beasley R P, Hwang L Y, Lee G C, Lan C C, Roan C H, Huang F Y, Chen C L. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet. 1983;2:1099–1102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI034266/AI/NIAID NIH HHS/United States
- KO8-AI01327/AI/NIAID NIH HHS/United States
- AI45320/AI/NIAID NIH HHS/United States
- R21 AI046177/AI/NIAID NIH HHS/United States
- P01 AI048240/AI/NIAID NIH HHS/United States
- P51 RR000165/RR/NCRR NIH HHS/United States
- R21 AI46177/AI/NIAID NIH HHS/United States
- P01 AI48240/AI/NIAID NIH HHS/United States
- N01 AI85343/AI/NIAID NIH HHS/United States
- AI26926/AI/NIAID NIH HHS/United States
- R01 AI026926/AI/NIAID NIH HHS/United States
- R01 AI34266/AI/NIAID NIH HHS/United States
- RR00165/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

