Reactivation of latent human cytomegalovirus in CD14(+) monocytes is differentiation dependent
- PMID: 11462026
- PMCID: PMC114989
- DOI: 10.1128/JVI.75.16.7543-7554.2001
Reactivation of latent human cytomegalovirus in CD14(+) monocytes is differentiation dependent
Abstract
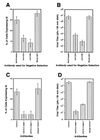
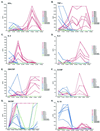
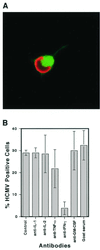
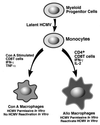
We have previously demonstrated reactivation of latent human cytomegalovirus (HCMV) in myeloid lineage cells obtained from healthy donors. Virus was obtained from allogenically stimulated monocyte-derived macrophages (Allo-MDM), but not from macrophages differentiated by mitogenic stimulation (ConA-MDM). In the present study, the cellular and cytokine components essential for HCMV replication and reactivation were examined in Allo-MDM. The importance of both CD4(+) and CD8(+) T cells in the generation of HCMV-permissive Allo-MDM was demonstrated by negative selection or blocking experiments using antibodies directed against both HLA class I and HLA class II molecules. Interestingly, contact of monocytes with CD4 or CD8 T cells was not essential for reactivation of HCMV, since virus was observed in macrophages derived from CD14(+) monocytes stimulated by supernatants produced by allogeneic stimulation of peripheral blood mononuclear cells. Examination of the cytokines produced in Allo-MDM and ConA-MDM cultures indicated a significant difference in the kinetics of production and quantity of these factors. Further examination of the cytokines essential for the generation of HCMV-permissive Allo-MDM identified gamma interferon (IFN-gamma) but not interleukin-1 or -2, tumor necrosis factor alpha, or granulocyte-macrophage colony-stimulating factor as critical components in the generation of these macrophages. In addition, although IFN-gamma was crucial for reactivation of latent HCMV, addition of IFN-gamma to unstimulated macrophage cultures was insufficient to reactivate virus. Thus, this study characterizes two distinct monocyte-derived cell types which can be distinguished by their ability to reactivate and support HCMV replication and identifies the critical importance of IFN-gamma in the reactivation of HCMV.
Figures




Similar articles
-
Growth of human cytomegalovirus in primary macrophages.Methods. 1998 Sep;16(1):126-38. doi: 10.1006/meth.1998.0650. Methods. 1998. PMID: 9774522
-
Interferon-gamma and tumor necrosis factor-alpha specifically induce formation of cytomegalovirus-permissive monocyte-derived macrophages that are refractory to the antiviral activity of these cytokines.J Clin Invest. 1997 Dec 15;100(12):3154-63. doi: 10.1172/JCI119871. J Clin Invest. 1997. PMID: 9399963 Free PMC article.
-
Human cytomegalovirus modulates monocyte-mediated innate immune responses during short-term experimental latency in vitro.J Virol. 2014 Aug;88(16):9391-405. doi: 10.1128/JVI.00934-14. Epub 2014 Jun 11. J Virol. 2014. PMID: 24920803 Free PMC article.
-
Human cytomegalovirus reactivation in bone-marrow-derived granulocyte/monocyte progenitor cells and mature monocytes.Intervirology. 1999;42(5-6):308-13. doi: 10.1159/000053965. Intervirology. 1999. PMID: 10702711 Review.
-
HCMV Infection and Apoptosis: How Do Monocytes Survive HCMV Infection?Viruses. 2018 Sep 29;10(10):533. doi: 10.3390/v10100533. Viruses. 2018. PMID: 30274264 Free PMC article. Review.
Cited by
-
A myeloid progenitor cell line capable of supporting human cytomegalovirus latency and reactivation, resulting in infectious progeny.J Virol. 2012 Sep;86(18):9854-65. doi: 10.1128/JVI.01278-12. Epub 2012 Jul 3. J Virol. 2012. PMID: 22761372 Free PMC article.
-
Latent Cytomegalovirus Infection in Female Mice Increases Breast Cancer Metastasis.Cancers (Basel). 2019 Mar 29;11(4):447. doi: 10.3390/cancers11040447. Cancers (Basel). 2019. PMID: 30934926 Free PMC article.
-
A Single-Cell Approach to the Elusive Latent Human Cytomegalovirus Transcriptome.mBio. 2018 Jun 12;9(3):e01001-18. doi: 10.1128/mBio.01001-18. mBio. 2018. PMID: 29895640 Free PMC article.
-
The Impact of Human Herpesviruses in Clinical Practice of Inflammatory Bowel Disease in the Era of COVID-19.Microorganisms. 2021 Sep 3;9(9):1870. doi: 10.3390/microorganisms9091870. Microorganisms. 2021. PMID: 34576764 Free PMC article. Review.
-
Congenital and Perinatal Viral Infections: Consequences for the Mother and Fetus.Viruses. 2024 Oct 30;16(11):1698. doi: 10.3390/v16111698. Viruses. 2024. PMID: 39599813 Free PMC article. Review.
References
-
- Bostrom L, Ringden O, Jacobsen N, Zwaan F, Nilsson B. A European multicenter study of chronic graft-versus-host disease: the role of cytomegalovirus serology in recipients and donors-acute graft-versus-host disease, and splenectomy. Transplantation. 1990;49:1100–1105. - PubMed
-
- Bowden R A. Transfusion-transmitted cytomegalovirus infection. Hematol Oncol Clin North Am. 1995;9:155–166. - PubMed
-
- Burns G, Begley C, Mackay I, Triglia R, Werkmeister J. Supernatural killer cells. Immunol Today. 1985;6:370–373. - PubMed
-
- Chou S, Kim D Y, Norman D J. Transmission of cytomegalovirus by pretransplant leukocyte transfusions in renal transplant candidates. J Infect Dis. 1987;155:565–567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

