Inhibition of p53 tumor suppressor by viral interferon regulatory factor
- PMID: 11462029
- PMCID: PMC114992
- DOI: 10.1128/JVI.75.16.7572-7582.2001
Inhibition of p53 tumor suppressor by viral interferon regulatory factor
Abstract
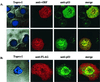
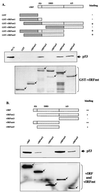
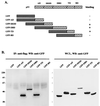
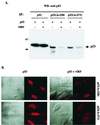
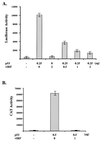
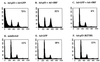
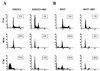
The irreversible cell cycle arrest and apoptosis induced by p53 are part of the host surveillance mechanisms for viral infection and tumor induction. Kaposi's sarcoma-associated herpesvirus (KSHV), the most recently discovered human tumor virus, is associated with the pathogenesis of Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. The K9 open reading frame of KSHV encodes a viral interferon (IFN) regulatory factor (vIRF) which functions as a repressor for cellular IFN-mediated signal transduction and as an oncoprotein to induce cell growth transformation. Here, we demonstrate that KSHV vIRF interacts with the cellular p53 tumor suppressor through the putative DNA binding region of vIRF and the central region of p53. This interaction suppresses the level of phosphorylation and acetylation of p53 and inhibits transcriptional activation of p53. As a consequence, vIRF efficiently prevents p53-mediated apoptosis. These results suggest that KSHV vIRF interacts with and inhibits the p53 tumor suppressor to circumvent host growth surveillance and to facilitate uncontrolled cell proliferation.
Figures



References
-
- Banin S, Moyal L, Shieh S, Taya Y, Anderson C W, Chessa L, Smorodinsky N I, Prives C, Reiss Y, Shiloh Y, Ziv Y. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. - PubMed
-
- Bates S, Hickman E S, Vousden K H. Reversal of p53-induced cell-cycle arrest. Mol Carcinog. 1999;24:7–14. - PubMed
-
- Cesarman E, Chang Y, Moore P S, Said J W, Knowles D M. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

