Duck hepatitis B virus replication in primary bile duct epithelial cells
- PMID: 11462037
- PMCID: PMC115000
- DOI: 10.1128/JVI.75.16.7651-7661.2001
Duck hepatitis B virus replication in primary bile duct epithelial cells
Abstract
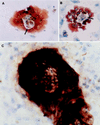
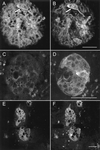
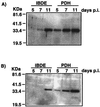
Primary cultures of intrahepatic bile duct epithelial (IBDE) cells isolated from duckling livers were successfully grown for studies of duck hepatitis B virus (DHBV). The primary IBDE cells were characterized by immunohistochemistry using CAM 5.2, a cytokeratin marker which was shown to react specifically to IBDE cells in duck liver tissue sections and in primary cultures of total duck liver cells. Immunofluorescence assay using anti-duck albumin, a marker for hepatocytes, revealed that these IBDE cultures did not appear to contain hepatocytes. A striking feature of these cultures was the duct-like structures present within each cell colony of multilayered IBDE cells. Normal duck serum in the growth medium was found to be essential for the development of these cells into duct-like structures. When the primary cultures of duck IBDE cells were acutely infected with DHBV, dual-labeled confocal microscopy using a combination of anti-DHBV core proteins and CAM 5.2 or a combination of anti-pre-S1 proteins and CAM 5.2 revealed that the IBDE cell colonies contained DHBV proteins. Immunoblot analysis of these cells showed that the DHBV pre-S1 and core proteins were similar to their counterparts in infected primary duck hepatocyte cultures. Southern blot analysis of infected IBDE preparations using a digoxigenin-labeled positive-sense DHBV riboprobe revealed the presence of hepadnavirus covalently closed circular (CCC) DNA, minus-sense single-stranded (SS) DNA, double-stranded linear DNA, and relaxed circular DNA. The presence of minus-sense SS DNA in the acutely infected IBDE cultures is indicative of DHBV reverse transcriptase activity, while the establishment of a pool of viral CCC DNA reveals the ability of these cells to maintain persistent infection. Taken collectively, the results from this study demonstrated that primary duck IBDE cells supported hepadnavirus replication as shown by the de novo synthesis of DHBV proteins and DNA replicative intermediates.
Figures



Similar articles
-
Effect of nucleoside analogue therapy on duck hepatitis B viral replication in hepatocytes and bile duct epithelial cells in vivo.J Gastroenterol Hepatol. 2000 Mar;15(3):304-10. doi: 10.1046/j.1440-1746.2000.02079.x. J Gastroenterol Hepatol. 2000. PMID: 10764033
-
Covalently closed circular DNA is the predominant form of duck hepatitis B virus DNA that persists following transient infection.J Virol. 2005 Oct;79(19):12242-52. doi: 10.1128/JVI.79.19.12242-12252.2005. J Virol. 2005. PMID: 16160150 Free PMC article.
-
The half-life of duck hepatitis B virus supercoiled DNA in congenitally infected primary hepatocyte cultures.Virology. 1994 Aug 15;203(1):81-9. doi: 10.1006/viro.1994.1457. Virology. 1994. PMID: 8030288
-
[Research on the gene structure of duck hepatitis B virus and its encoding proteins].Bing Du Xue Bao. 2012 Nov;28(6):681-8. Bing Du Xue Bao. 2012. PMID: 23367570 Review. Chinese.
-
Duck hepatitis B virus infection, aflatoxin B1 and liver cancer in ducks.Arch Virol Suppl. 1993;8:81-7. doi: 10.1007/978-3-7091-9312-9_9. Arch Virol Suppl. 1993. PMID: 8260880 Review.
Cited by
-
Entry of duck hepatitis B virus into primary duck liver and kidney cells after discovery of a fusogenic region within the large surface protein.J Virol. 2007 May;81(10):5014-23. doi: 10.1128/JVI.02290-06. Epub 2007 Mar 14. J Virol. 2007. PMID: 17360753 Free PMC article.
-
Hepatitis B virus-related intrahepatic cholangiocarcinoma originates from hepatocytes.Hepatol Int. 2023 Oct;17(5):1300-1317. doi: 10.1007/s12072-023-10556-3. Epub 2023 Jun 27. Hepatol Int. 2023. PMID: 37368186 Free PMC article.
-
Entecavir therapy combined with DNA vaccination for persistent duck hepatitis B virus infection.Antimicrob Agents Chemother. 2003 Aug;47(8):2624-35. doi: 10.1128/AAC.47.8.2624-2635.2003. Antimicrob Agents Chemother. 2003. PMID: 12878529 Free PMC article.
-
Endocytosis of hepatitis B immune globulin into hepatocytes inhibits the secretion of hepatitis B virus surface antigen and virions.J Virol. 2003 Aug;77(16):8882-92. doi: 10.1128/jvi.77.16.8882-8892.2003. J Virol. 2003. PMID: 12885906 Free PMC article.
References
-
- Alpini G, Phillips J O, Vroman B, LaRusso N F. Recent advances in the isolation of liver cells. Hepatology. 1994;20:494–514. - PubMed
-
- Beasley R P, Hwang L Y, Lin C C, Chien C S. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22707 men in Taiwan. Lancet. 1981;ii:1129–1133. - PubMed
-
- Birnbaum A, Suchy F J. The intrahepatic cholangiopathies. Semin Liver Dis. 1998;18:263–269. - PubMed
-
- Bishop N, Civitico G, Wang Y Y, Guo K J, Birch C, Gust I, Locarnini S. Antiviral strategies in chronic hepatitis B virus infection: I. Establishment of an in vitro system using the duck hepatitis B virus model. J Med Virol. 1990;31:82–89. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

