Effective human herpesvirus 8 infection of human umbilical vein endothelial cells by cell-mediated transmission
- PMID: 11462044
- PMCID: PMC115007
- DOI: 10.1128/JVI.75.16.7717-7722.2001
Effective human herpesvirus 8 infection of human umbilical vein endothelial cells by cell-mediated transmission
Abstract
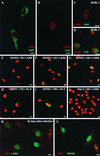
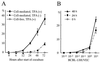
Cell-free transmission of human herpesvirus 8 (HHV-8) to human cells in vitro has been reported to be difficult, if not impossible. The present experiments were conducted with the idea that cell-cell contact may produce much more effective transmission, so-called cell-mediated transmission. Primary human umbilical vein endothelial cells (HUVECs) were cocultured with an HHV-8-infected lymphoma cell line, BCBL-1 cells. When a ratio of 12-O-tetradecanoylphorbol-13-acetate (TPA)-treated BCBL-1 cells to HUVECs of 10:1 was used, more than 20% of HUVECs were found to express the HHV-8 latency-associated nuclear antigen (LANA) 48 h after the start of coculturing; this value increased to more than 30% after 72 h. HHV-8-encoded ORF26, K8, K8.1, K10, K11, ORF59, and ORF65 proteins were not detected in these HHV-8-infected HUVECs until 72 h. The HHV-8 antigens were not observed in HUVECs cocultured with TPA-treated BCBL-1 cells separated by a membrane. Thirty days after removal of the BCBL-1 cells from the cell-mediated transmission experiment, the HUVECs still expressed LANA and the HHV-8 genome was detected by PCR in these cells. Moreover, the ORF59 protein, a DNA replication-associated protein of HHV-8, was expressed in such HUVECs in the presence of TPA stimulation. These results indicated a far more effective transmission mechanism, cell-cell contact, suggesting the possibility that such a mechanism works in vivo.
Figures

Similar articles
-
Retinoic acid analogues inhibit human herpesvirus 8 replication.Antivir Ther. 2008;13(2):199-209. Antivir Ther. 2008. PMID: 18505171
-
Identification and characterization of human herpesvirus-8 lytic cycle-associated ORF 59 protein and the encoding cDNA by monoclonal antibody.Virology. 1998 Jan 5;240(1):118-26. doi: 10.1006/viro.1997.8911. Virology. 1998. PMID: 9448696
-
Human interleukin-6 induces human herpesvirus-8 replication in a body cavity-based lymphoma cell line.J Med Virol. 2002 Nov;68(3):404-11. doi: 10.1002/jmv.10218. J Med Virol. 2002. PMID: 12226829
-
Optimization of chemical induction conditions for human herpesvirus 8 (HHV-8) reactivation with 12-O-tetradecanoyl-phorbol-13-acetate (TPA) from latently-infected BC-3 cells.Biologicals. 2011 May;39(3):158-66. doi: 10.1016/j.biologicals.2011.03.001. Epub 2011 Apr 5. Biologicals. 2011. PMID: 21470875
-
HHV-8 encoded LANA-1 alters the higher organization of the cell nucleus.Mol Cancer. 2007 Apr 13;6:28. doi: 10.1186/1476-4598-6-28. Mol Cancer. 2007. PMID: 17433107 Free PMC article.
Cited by
-
Virus-Driven Carcinogenesis.Cancers (Basel). 2021 May 27;13(11):2625. doi: 10.3390/cancers13112625. Cancers (Basel). 2021. PMID: 34071792 Free PMC article. Review.
-
The latency-associated nuclear antigen of rhesus monkey rhadinovirus inhibits viral replication through repression of Orf50/Rta transcriptional activation.J Virol. 2005 Mar;79(5):3127-38. doi: 10.1128/JVI.79.5.3127-3138.2005. J Virol. 2005. PMID: 15709032 Free PMC article.
-
Surface downregulation of major histocompatibility complex class I, PE-CAM, and ICAM-1 following de novo infection of endothelial cells with Kaposi's sarcoma-associated herpesvirus.J Virol. 2003 Sep;77(17):9669-84. doi: 10.1128/jvi.77.17.9669-9684.2003. J Virol. 2003. PMID: 12915579 Free PMC article.
-
Disruption of LANA in rhesus rhadinovirus generates a highly lytic recombinant virus.J Virol. 2009 Oct;83(19):9786-802. doi: 10.1128/JVI.00704-09. Epub 2009 Jul 8. J Virol. 2009. PMID: 19587030 Free PMC article.
-
Whole-genome transcription profiling of rhesus monkey rhadinovirus.J Virol. 2005 Jul;79(13):8637-50. doi: 10.1128/JVI.79.13.8637-8650.2005. J Virol. 2005. PMID: 15956606 Free PMC article.
References
-
- Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. - PubMed
-
- Chatlynne L G, Lapps W, Handy M, Huang Y Q, Masood R, Hamilton A S, Said J W, Koeffler H P, Kaplan M H, Friedman K A, Gill P S, Whitman J E, Ablashi D V. Detection and titration of human herpesvirus-8-specific antibodies in sera from blood donors, acquired immunodeficiency syndrome patients, and Kaposi's sarcoma patients using a whole virus enzyme-linked immunosorbent assay. Blood. 1998;92:53–58. - PubMed
-
- Corbeil J, Evans L A, Vasak E, Cooper D A, Penny R. Culture and properties of cells derived from Kaposi's sarcoma. J Immunol. 1991;146:2972–2976. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

