The A3 adenosine receptor induces cytoskeleton rearrangement in human astrocytoma cells via a specific action on Rho proteins
- PMID: 11462805
- PMCID: PMC4804712
- DOI: 10.1111/j.1749-6632.2001.tb03613.x
The A3 adenosine receptor induces cytoskeleton rearrangement in human astrocytoma cells via a specific action on Rho proteins
Abstract
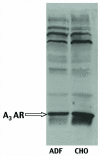
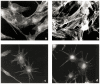
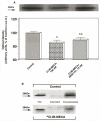
In previous studies, we have demonstrated that exposure of astroglial cells to A3 adenosine receptor agonists results in dual actions on cell survival, with "trophic" and antiapoptotic effects at nanomolar concentrations and induction of cell death at micromolar agonist concentrations. The protective actions of A3 agonists have been associated with a reinforcement of the actin cytoskeleton, which likely results in increased resistance of cells to cytotoxic stimuli. The molecular mechanisms at the basis of this effect and the signalling pathway(s) linking the A3 receptor to the actin cytoskeleton have never been elucidated. Based on previous literature data suggesting that the actin cytoskeleton is controlled by small GTP-binding proteins of the Rho family, in the study reported here we investigated the involvement of these proteins in the effects induced by A3 agonists on human astrocytoma ADF cells. The presence of the A3 adenosine receptor in these cells has been confirmed by immunoblotting analysis. As expected, exposure of human astrocytoma ADF cells to nanomolar concentrations of the selective A3 agonist 2-chloro-N6-(3-iodobenzyl)-adenosine-5'-N-methyluronamide (CI-IB-MECA) resulted in formation of thick actin positive stress fibers. Preexposure of cells to the C3B toxin that inactivates Rho-proteins completely prevented the actin changes induced by CI-IB-MECA. Exposure to the A3 agonist also resulted in significant reduction of Rho-GDI, an inhibitory protein known to maintain Rho proteins in their inactive state, suggesting a potentiation of Rho-mediated effects. This effect was fully counteracted by the concomitant exposure to the selective A3 receptor antagonist MRS1191. These results suggest that the reinforcement of the actin cytoskeleton induced by A3 receptor agonists is mediated by an interference with the activation/inactivation cycle of Rho proteins, which may, therefore, represent a biological target for the identification of novel neuroprotective strategies.
Figures
Similar articles
-
The A3 adenosine receptor mediates cell spreading, reorganization of actin cytoskeleton, and distribution of Bcl-XL: studies in human astroglioma cells.Biochem Biophys Res Commun. 1997 Dec 18;241(2):297-304. doi: 10.1006/bbrc.1997.7705. Biochem Biophys Res Commun. 1997. PMID: 9425266 Free PMC article.
-
Adenosine A3 Receptors and Viability of Astrocytes.Drug Dev Res. 1998 Nov-Dec;45(3-4):379-386. doi: 10.1002/(sici)1098-2299(199811/12)45:3/4<379::aid-ddr38>3.0.co;2-y. Drug Dev Res. 1998. PMID: 38239500 Free PMC article.
-
Activation of the A3 adenosine receptor affects cell cycle progression and cell growth.Naunyn Schmiedebergs Arch Pharmacol. 2000 Mar;361(3):225-34. doi: 10.1007/s002109900186. Naunyn Schmiedebergs Arch Pharmacol. 2000. PMID: 10731034 Free PMC article.
-
Effects of synthetic A3 adenosine receptor agonists on cell proliferation and viability are receptor independent at micromolar concentrations.J Physiol Biochem. 2013 Sep;69(3):405-17. doi: 10.1007/s13105-012-0222-7. Epub 2012 Nov 27. J Physiol Biochem. 2013. PMID: 23184730
-
A3 adenosine receptor as a target for cancer therapy.Anticancer Drugs. 2002 Jun;13(5):437-43. doi: 10.1097/00001813-200206000-00001. Anticancer Drugs. 2002. PMID: 12045454 Review.
Cited by
-
A3 Adenosine and P2X7 Purinergic Receptors as New Targets for an Innovative Pharmacological Therapy of Malignant Pleural Mesothelioma.Front Oncol. 2021 Oct 1;11:679285. doi: 10.3389/fonc.2021.679285. eCollection 2021. Front Oncol. 2021. PMID: 34660262 Free PMC article.
-
14-3-3σ attenuates RhoGDI2-induced cisplatin resistance through activation of Erk and p38 in gastric cancer cells.Oncotarget. 2013 Nov;4(11):2045-56. doi: 10.18632/oncotarget.1334. Oncotarget. 2013. PMID: 24185104 Free PMC article.
-
The Anti-Cancer Effect of A3 Adenosine Receptor Agonists: A Novel, Targeted Therapy.Immunol Endocr Metab Agents Med Chem. 2007 Aug;7:298-303. doi: 10.2174/187152207781369878. Immunol Endocr Metab Agents Med Chem. 2007. PMID: 34824647 Free PMC article.
-
Purinergic signalling and cancer.Purinergic Signal. 2013 Dec;9(4):491-540. doi: 10.1007/s11302-013-9372-5. Purinergic Signal. 2013. PMID: 23797685 Free PMC article. Review.
-
A3 adenosine receptors in human astrocytoma cells: agonist-mediated desensitization, internalization, and down-regulation.Mol Pharmacol. 2002 Dec;62(6):1373-84. doi: 10.1124/mol.62.6.1373. Mol Pharmacol. 2002. PMID: 12435805 Free PMC article.
References
-
- LINDEN J. Cloned adenosine A3 receptors: pharmacological properties, species-differences and receptor functions. Trends Pharmacol. Sci. 1994;15:298–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

