Sex and sugar in yeast: two distinct GPCR systems
- PMID: 11463740
- PMCID: PMC1083946
- DOI: 10.1093/embo-reports/kve132
Sex and sugar in yeast: two distinct GPCR systems
Abstract
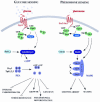
Although eukaryotic G-protein coupled receptor (GPCR) systems are well known for their ability to detect and mediate rapid responses to extracellular signals, the full range of stimuli to which they respond may not yet have been identified. Activation of GPCRs by hormones, pheromones, odorants, neurotransmitters, light and different taste compounds is well established. However, the recent discovery of a glucose-sensing GPCR system in Saccharomyces cerevisiae has unexpectedly added common nutrients to this list of stimuli. This GPCR system mediates glucose activation of adenylate cyclase during the switch from respirative/gluconeogenic metabolism to fermentation. The GPCR system involved in pheromone signalling in S. cerevisiae has already served as an important model and tool for the study of GPCR systems in higher eukaryotic cell types. Here, we highlight the similarities and differences between these two signalling systems. We also indicate how the new glucose-sensing system can serve as a model for GPCR function and as a tool with which to screen for heterologous components of signalling pathways as well as for novel ligands in high-throughput assays.
Figures




Similar articles
-
The N-terminal non-RGS domain of human regulator of G-protein signalling 1 contributes to its ability to inhibit pheromone receptor signalling in yeast.Cell Signal. 2003 Apr;15(4):413-21. doi: 10.1016/s0898-6568(02)00121-3. Cell Signal. 2003. PMID: 12618216
-
A Saccharomyces cerevisiae G-protein coupled receptor, Gpr1, is specifically required for glucose activation of the cAMP pathway during the transition to growth on glucose.Mol Microbiol. 1999 Jun;32(5):1002-12. doi: 10.1046/j.1365-2958.1999.01413.x. Mol Microbiol. 1999. PMID: 10361302
-
Coactivation of G protein signaling by cell-surface receptors and an intracellular exchange factor.Curr Biol. 2008 Feb 12;18(3):211-5. doi: 10.1016/j.cub.2008.01.007. Curr Biol. 2008. PMID: 18261907 Free PMC article.
-
Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae.Mol Microbiol. 1999 Sep;33(5):904-18. doi: 10.1046/j.1365-2958.1999.01538.x. Mol Microbiol. 1999. PMID: 10476026 Review.
-
Yeast assays for G-protein-coupled receptors.Recept Channels. 2002;8(5-6):343-52. Recept Channels. 2002. PMID: 12690961 Review.
Cited by
-
AaPKAc Regulates Differentiation of Infection Structures Induced by Physicochemical Signals From Pear Fruit Cuticular Wax, Secondary Metabolism, and Pathogenicity of Alternaria alternata.Front Plant Sci. 2021 Apr 21;12:642601. doi: 10.3389/fpls.2021.642601. eCollection 2021. Front Plant Sci. 2021. PMID: 33968101 Free PMC article.
-
Fungal Stress Responses and the Importance of GPCRs.J Fungi (Basel). 2025 Mar 11;11(3):213. doi: 10.3390/jof11030213. J Fungi (Basel). 2025. PMID: 40137251 Free PMC article. Review.
-
Gip1 GPCR regulates two sexual-stage differentiation processes in the ascomycete Fusarium graminearum.Nat Commun. 2025 Jul 8;16(1):6279. doi: 10.1038/s41467-025-61704-2. Nat Commun. 2025. PMID: 40628729 Free PMC article.
-
Functional coupling of a nematode chemoreceptor to the yeast pheromone response pathway.PLoS One. 2014 Nov 21;9(11):e111429. doi: 10.1371/journal.pone.0111429. eCollection 2014. PLoS One. 2014. PMID: 25415379 Free PMC article.
-
Acyl coenzyme A binding protein (ACBP): An aging- and disease-relevant "autophagy checkpoint".Aging Cell. 2023 Sep;22(9):e13910. doi: 10.1111/acel.13910. Epub 2023 Jun 26. Aging Cell. 2023. PMID: 37357988 Free PMC article. Review.
References
-
- Berghuis A.M., Lee, E., Raw, A.S., Gilman, A.G. and Sprang, S.R. (1996) Structure of the GDP-Pi complex of Gly203→Ala giα1: a mimic of the ternary product complex of gα-catalyzed GTP hydrolysis. Structure, 4, 1277–1290. - PubMed
-
- Beullens M., Mbonyi, K., Geerts, L., Gladines, D., Detremerie, K., Jans, A.W.H. and Thevelein, J.M. (1988) Studies on the mechanism of the glucose-induced cAMP signal in glycolysis and glucose repression mutants of the yeast Saccharomyces cerevisiae. Eur. J. Biochem., 172, 227–231. - PubMed
-
- Brown A.J. et al. (2000) Functional coupling of mammalian receptors to the yeast mating pathway using novel yeast/mammalian G protein α-subunit chimeras. Yeast, 16, 11–22. - PubMed
-
- Cismowski M.J., Takesono, A., Ma, C., Lizano, J.S., Xie, X., Fuernkranz, H., Lanier, S.M. and Duzic, E. (1999) Genetic screens in yeast to identify mammalian nonreceptor modulators of G-protein signaling. Nature Biotechnol., 17, 878–883. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

