Structure--function relationships in HIV-1 Nef
- PMID: 11463741
- PMCID: PMC1083955
- DOI: 10.1093/embo-reports/kve141
Structure--function relationships in HIV-1 Nef
Abstract
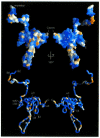
The accessory Nef protein of HIV and SIV is essential for viral pathogenesis, yet it is perplexing in its multitude of molecular functions. In this review we analyse the structure-function relationships of motifs recently proposed to play roles in aspects of Nef modification, signalling and trafficking, and thereby to impinge on the ability of the virus to survive in, and to manipulate, its cellular host. Based on the full-length structure assembly of HIV Nef, we correlate surface accessibility with secondary structure elements and sequence conservation. Motifs involved in Nef-mediated CD4 and MHC I downregulation are located in flexible regions of Nef, suggesting that the formation of the transient trafficking complexes involved in these processes depends on the recognition of primary sequences. In contrast, the interaction sites for signalling molecules that contain SH3 domains or the p21-activated kinases are associated with the well folded core domain, suggesting the recognition of highly structured protein surfaces.
Figures




References
-
- Aiken C., Krause, L., Chen, Y.-L. and Trono, D. (1996) Mutational analysis of HIV-1 Nef: identification of two mutants that are temperature-sensitive for CD4 downregulation. Virology, 217, 293–300. - PubMed
-
- Arold S., Franken, P., Strub, M.-P., Hoh, F., Benichou, S., Benarous, R. and Dumas, C. (1997) The crystal structure of HIV-1 Nef protein bound to the Fyn kinase SH3 domain suggests a role for this complex in altered T cell receptor signaling. Structure, 5, 1361–1372. - PubMed
-
- Benichou S., Bomsel, M., Bodéus, M., Durand, H., Douté, M., Letourneur, F., Camonis, J. and Benarous, R. (1994) Physical interaction of the HIV-1 Nef protein with β-COP, a component of non-clathrin-coated vesicles essential for membrane traffic. J. Biol. Chem., 269, 30073–30076. - PubMed
-
- Bresnahan P.A., Yonemoto, W., Ferrell, S., Williams-Herman, D., Geleziunas, R. and Greene, W.C. (1998) A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr. Biol., 8, 1235–1238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

