CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri
- PMID: 11463746
- PMCID: PMC1083992
- DOI: 10.1093/embo-reports/kve155
CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri
Abstract
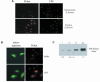
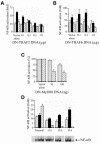
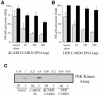
Epithelial cells are refractory to extracellular lipopolysaccharide (LPS), yet when presented inside the cell, it is capable of initiating an inflammatory response. Using invasive Shigella flexneri to deliver LPS into the cytosol, we examined how this factor, once intracellular, activates both NF-kappaB and c-Jun N-terminal kinase (JNK). Surprisingly, the mode of activation is distinct from that induced by toll-like receptors (TLRs), which mediate LPS responsiveness from the outside-in. Instead, our findings demonstrate that this response is mediated by a cytosolic, plant disease resistance-like protein called CARD4/Nod1. Biochemical studies reveal enhanced oligomerization of CARD4 upon S. flexneri infection, an event necessary for NF-kappaB induction. Dominant-negative versions of CARD4 block activation of NF-kappaB and JNK by S. flexneri as well as microinjected LPS. Finally, we showed that invasive S. flexneri triggers the formation of a transient complex involving CARD4, RICK and the IKK complex. This study demonstrates that in addition to the extracellular LPS sensing system mediated by TLRs, mammalian cells also possess a cytoplasmic means of LPS detection via a molecule that is related to plant disease-resistance proteins.
Figures


References
-
- Bertin J. and Distefano, P.S. (2000) The PYRIN domain: A novel motif found in apoptosis and inflammation proteins. Cell Death Differ., 7, 1273–1274. - PubMed
-
- Bertin J. et al. (1999) Human CARD4 protein is a novel CED-4/Apaf-1 cell death family member that activates NF-κB. J. Biol. Chem., 274, 12955–12958. - PubMed
-
- Cao Z., Xiong, J., Takeuchi, M., Kurama, T. and Goeddel, D.V. (1996) TRAF6 is a signal transducer for interleukin-1. Nature, 383, 443–446. - PubMed
-
- Cario E., Rosenberg, I.M., Brandwein, S.L., Beck, P.L., Reinecker, H.C. and Podolsky, D.K. (2000) Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol., 164, 966–972. - PubMed
-
- Foletta V.C., Segal, D.H. and Cohen, D.R. (1998) Transcriptional regulation in the immune system: all roads lead to AP-1. J. Leukoc. Biol., 63, 139–152. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

