Inhibition of the motility and growth of B16F10 mouse melanoma cells by dominant negative mutants of Dok-1
- PMID: 11463826
- PMCID: PMC87266
- DOI: 10.1128/MCB.21.16.5437-5446.2001
Inhibition of the motility and growth of B16F10 mouse melanoma cells by dominant negative mutants of Dok-1
Abstract
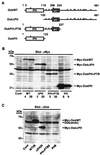
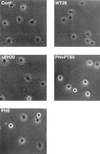
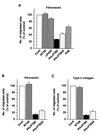
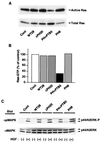
Dok-1 (p62(Dok)) is a multiple-site docking protein that acts downstream of receptor and nonreceptor tyrosine kinases. Although it has been proposed to contribute to the control of cell growth and migration through association with the Ras GTPase-activating protein and the adapter protein Nck, the role of Dok-1 remains largely unknown. The functions of Dok-1 have now been investigated by the generation of two different COOH-terminal truncation mutants of this protein: one (DokPH+PTB) containing the pleckstrin homology and phosphotyrosine-binding domains, and the other (DokPH) composed only of the pleckstrin homology domain. Both of these mutant proteins were shown to act in a dominant negative manner. Overexpression of each of the mutants in highly metastatic B16F10 mouse melanoma cells thus both inhibited the tyrosine phosphorylation of endogenous Dok-1 induced by cell adhesion as well as reduced the association of the endogenous protein with cellular membranes and the cytoskeleton. Overexpression of DokPH+PTB in these cells also markedly reduced both the rates of cell spreading, migration, and growth as well as the extent of Ras activation. The effects of DokPH on these processes were less pronounced than were those of DokPH+PTB, indicating the importance of the phosphotyrosine-binding domain. These results suggest that at least in B16F10 cells, Dok-1 positively regulates not only cell spreading and migration but also cell growth and Ras activity.
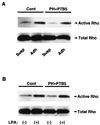
Figures




Similar articles
-
Tyrosine phosphorylation of p62(Dok) induced by cell adhesion and insulin: possible role in cell migration.EMBO J. 1999 Apr 1;18(7):1748-60. doi: 10.1093/emboj/18.7.1748. EMBO J. 1999. PMID: 10202139 Free PMC article.
-
Dok-1 tyrosine residues at 336 and 340 are essential for the negative regulation of Ras-Erk signalling, but dispensable for rasGAP-binding.Genes Cells. 2004 Jun;9(6):601-7. doi: 10.1111/j.1356-9597.2004.00748.x. Genes Cells. 2004. PMID: 15189452
-
The Tek/Tie2 receptor signals through a novel Dok-related docking protein, Dok-R.Oncogene. 1998 Sep 3;17(9):1097-108. doi: 10.1038/sj.onc.1202115. Oncogene. 1998. PMID: 9764820
-
The roles of Dok family adapters in immunoreceptor signaling.Immunol Rev. 2009 Nov;232(1):273-85. doi: 10.1111/j.1600-065X.2009.00844.x. Immunol Rev. 2009. PMID: 19909370 Review.
-
Dok protein family members are involved in signaling mediated by the type 1 Fcepsilon receptor.Eur J Immunol. 2003 Jan;33(1):85-91. doi: 10.1002/immu.200390011. Eur J Immunol. 2003. PMID: 12594836
Cited by
-
Chemical groups that adhere to the surfaces of living malignant cells.Pharm Res. 2007 Dec;24(12):2370-80. doi: 10.1007/s11095-007-9436-8. Epub 2007 Sep 12. Pharm Res. 2007. PMID: 17849176
-
Subcellular compartmentalization of docking protein-1 contributes to progression in colorectal cancer.EBioMedicine. 2016 Jun;8:159-172. doi: 10.1016/j.ebiom.2016.05.003. Epub 2016 May 5. EBioMedicine. 2016. PMID: 27428427 Free PMC article.
-
Atomic force microscopy study of the specific adhesion between a colloid particle and a living melanoma cell: Effect of the charge and the hydrophobicity of the particle surface.Biophys J. 2006 Sep 1;91(5):1960-9. doi: 10.1529/biophysj.106.082420. Epub 2006 May 26. Biophys J. 2006. PMID: 16731555 Free PMC article.
-
Advances in targeting IKK and IKK-related kinases for cancer therapy.Clin Cancer Res. 2008 Sep 15;14(18):5656-62. doi: 10.1158/1078-0432.CCR-08-0123. Clin Cancer Res. 2008. PMID: 18794072 Free PMC article. Review.
-
IkappaB kinase beta phosphorylates Dok1 serines in response to TNF, IL-1, or gamma radiation.Proc Natl Acad Sci U S A. 2004 Dec 14;101(50):17416-21. doi: 10.1073/pnas.0408061101. Epub 2004 Dec 1. Proc Natl Acad Sci U S A. 2004. PMID: 15574499 Free PMC article.
References
-
- Bouton A H, Kanner S B, Vines R R, Wang H C, Gibbs J B, Parsons J T. Transformation by pp60src or stimulation of cells with epidermal growth factor induces the stable association of tyrosine-phosphorylated cellular proteins with GTPase-activating protein. Mol Cell Biol. 1991;11:945–953. - PMC - PubMed
-
- Carpino N, Wisniewski D, Strife A, Marshak D, Kobayashi R, Stillman B, Clarkson B. p62(dok): a constitutively tyrosine-phosphorylated, GAP-associated protein in chronic myelogenous leukemia progenitor cells. Cell. 1997;88:197–204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
