Dichotomy of AML1-ETO functions: growth arrest versus block of differentiation
- PMID: 11463839
- PMCID: PMC87279
- DOI: 10.1128/MCB.21.16.5577-5590.2001
Dichotomy of AML1-ETO functions: growth arrest versus block of differentiation
Abstract
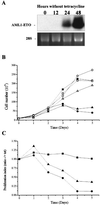
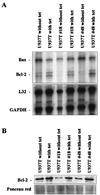
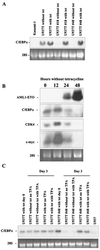
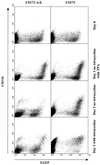
The fusion gene AML1-ETO is the product of t(8;21)(q22;q22), one of the most common chromosomal translocations associated with acute myeloid leukemia. To investigate the impact of AML1-ETO on hematopoiesis, tetracycline-inducible AML1-ETO-expressing cell lines were generated using myeloid cells. AML1-ETO is tightly and strongly induced upon tetracycline withdrawal. The proliferation of AML1-ETO(+) cells was markedly reduced, and most of the cells eventually underwent apoptosis. RNase protection assays revealed that the amount of Bcl-2 mRNA was decreased after AML1-ETO induction. Enforced expression of Bcl-2 was able to significantly delay, but not completely overcome, AML1-ETO-induced apoptosis. Prior to the onset of apoptosis, we also studied the ability of AML1-ETO to modulate differentiation. AML1-ETO expression altered granulocytic differentiation of U937T-A/E cells. More significantly, this change of differentiation was associated with the down-regulation of CCAAT/enhancer binding protein alpha (C/EBPalpha), a key regulator of granulocytic differentiation. These observations suggest a dichotomy in the functions of AML1-ETO: (i) reduction of granulocytic differentiation correlated with decreased expression of C/EBPalpha and (ii) growth arrest leading to apoptosis with decreased expression of CDK4, c-myc, and Bcl-2. We predict that the preleukemic AML1-ETO(+) cells must overcome AML1-ETO-induced growth arrest and apoptosis prior to fulfilling their leukemogenic potential.
Figures






References
-
- Ahn M Y, Huang G, Bae S C, Wee H J, Kim W Y, Ito Y. Negative regulation of granulocytic differentiation in the myeloid precursor cell line 32Dcl3 by ear-2, a mammalian homolog of Drosophila seven-up, and a chimeric leukemogenic gene, AML1/ETO. Proc Natl Acad Sci USA. 1998;95:1812–1817. - PMC - PubMed
-
- Banker D E, Radich J, Becker A, Kerkof K, Norwood T, Willman C, Appelbaum F R. The t(8;21) translocation is not consistently associated with high Bcl-2 expression in de novo acute myeloid leukemias of adults. Clin Cancer Res. 1998;4:3051–3062. - PubMed
-
- Borner C. Diminished cell proliferation associated with the death-protective activity of Bcl-2. J Biol Chem. 1996;271:12695–12698. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
