Structure-function analysis of BfpB, a secretin-like protein encoded by the bundle-forming-pilus operon of enteropathogenic Escherichia coli
- PMID: 11466288
- PMCID: PMC99539
- DOI: 10.1128/JB.183.16.4848-4859.2001
Structure-function analysis of BfpB, a secretin-like protein encoded by the bundle-forming-pilus operon of enteropathogenic Escherichia coli
Abstract
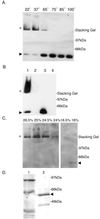
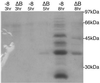
Production of type IV bundle-forming pili by enteropathogenic Escherichia coli (EPEC) requires BfpB, an outer-membrane lipoprotein and member of the secretin protein superfamily. BfpB was found to compose a ring-shaped, high-molecular-weight outer-membrane complex that is stable in 4% sodium dodecyl sulfate at temperatures of < or = 65 degrees C. Chemical cross-linking and immunoprecipitation experiments disclosed that the BfpB multimeric complex interacts with BfpG, and mutational studies showed that BfpG is required for the formation and/or stability of the multimer but not for the outer-membrane localization of BfpB. Formation of the BfpB multimer also does not require BfpA, the repeating subunit of the pilus filament. Functional studies of the BfpB-BfpG complex revealed that its presence confers vancomycin sensitivity, indicating that it may form an incompletely gated channel through the outer membrane. BfpB expression is also associated with accumulation of EPEC proteins in growth medium, suggesting that it may support both pilus biogenesis and protein secretion.
Figures






References
-
- Bieber D, Ramer S W, Wu C Y, Murray W J, Tobe T, Fernandez R, Schoolnik G K. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science. 1998;280:2114–2118. - PubMed
-
- Bitter W, Koster M, Latijnhouwers M, de Cock H, Tommassen J. Formation of oligomeric rings by XcpQ and PilQ, which are involved in protein transport across the outer membrane of Pseudomonas aeruginosa. Mol Microbiol. 1998;27:209–219. - PubMed
-
- Bitter W, Tommassen J. Ushers and other doorkeepers. Trends Microbiol. 1999;7:4–6. , 6–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

