Gap junctions mediate electrical signaling and ensuing cytosolic Ca2+ increases between chromaffin cells in adrenal slices: A role in catecholamine release
- PMID: 11466411
- PMCID: PMC6762648
- DOI: 10.1523/JNEUROSCI.21-15-05397.2001
Gap junctions mediate electrical signaling and ensuing cytosolic Ca2+ increases between chromaffin cells in adrenal slices: A role in catecholamine release
Abstract
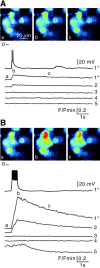
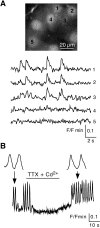
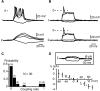
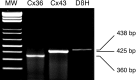
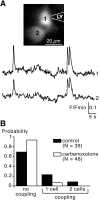
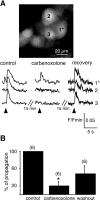
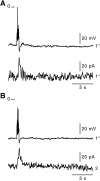
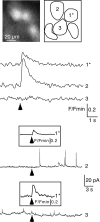
In adrenal chromaffin cells, a rise in cytosolic calcium concentration ([Ca(2+)]i) is a key event in the triggering of catecholamine exocytosis after splanchnic nerve activation. Action potential- or nicotine-induced [Ca(2+)]i transients are well described in individual chromaffin cells, but whether they remain spatially confined to the stimulated cell or propagate to adjacent cells is not yet known. To address this issue, the spatiotemporal organization of electrical and associated Ca(2+) events between chromaffin cells was investigated using the patch-clamp technique and real-time confocal imaging in rat acute adrenal slices. Spontaneous or electrically evoked action potential-driven [Ca(2+)]i transients were simultaneously detected in neighboring cells. This was likely attributable to gap junction-mediated electrotonic communication, as shown by (1) the bidirectional reflection of voltage changes monitored between cell pairs, (2) Lucifer yellow (LY) diffusion between cells exhibiting spontaneous synchronized [Ca(2+)]i transients, and (3) the reduction of LY diffusion using the uncoupling agent carbenoxolone. Furthermore, transcripts encoding two connexins (Cx36 and Cx43) were found in single chromaffin cells. This gap junctional coupling was activated after a synaptic-like application of nicotine that mediated synchronous multicellular [Ca(2+)]i increases. In addition, nicotinic stimulation of a single cell triggered catecholamine release in coupled cells, as shown by amperometric detection of secretory events. Functional coupling between chromaffin cells in situ may represent an efficient complement to synaptic transmission to amplify catecholamine release after synaptic stimulation of a single excited chromaffin cell.
Figures
Similar articles
-
Functional remodeling of gap junction-mediated electrical communication between adrenal chromaffin cells in stressed rats.J Neurosci. 2008 Jun 25;28(26):6616-26. doi: 10.1523/JNEUROSCI.5597-07.2008. J Neurosci. 2008. PMID: 18579734 Free PMC article.
-
Evidence for long-lasting cholinergic control of gap junctional communication between adrenal chromaffin cells.J Neurosci. 2003 May 1;23(9):3669-78. doi: 10.1523/JNEUROSCI.23-09-03669.2003. J Neurosci. 2003. PMID: 12736338 Free PMC article.
-
Therapeutic concentrations of varenicline increases exocytotic release of catecholamines from human and rat adrenal chromaffin cells in the presence of nicotine.Neuropharmacology. 2021 Sep 1;195:108632. doi: 10.1016/j.neuropharm.2021.108632. Epub 2021 Jun 4. Neuropharmacology. 2021. PMID: 34097947
-
Functional chromaffin cell plasticity in response to stress: focus on nicotinic, gap junction, and voltage-gated Ca2+ channels.J Mol Neurosci. 2012 Oct;48(2):368-86. doi: 10.1007/s12031-012-9707-7. Epub 2012 Jan 18. J Mol Neurosci. 2012. PMID: 22252244 Free PMC article. Review.
-
Gap junction communication between chromaffin cells: the hidden face of adrenal stimulus-secretion coupling.Pflugers Arch. 2018 Jan;470(1):89-96. doi: 10.1007/s00424-017-2032-9. Epub 2017 Jul 22. Pflugers Arch. 2018. PMID: 28735418 Review.
Cited by
-
Functional characterization of alpha9-containing cholinergic nicotinic receptors in the rat adrenal medulla: implication in stress-induced functional plasticity.J Neurosci. 2010 May 12;30(19):6732-42. doi: 10.1523/JNEUROSCI.4997-09.2010. J Neurosci. 2010. PMID: 20463235 Free PMC article.
-
Spatially Organized β-Cell Subpopulations Control Electrical Dynamics across Islets of Langerhans.Biophys J. 2017 Sep 5;113(5):1093-1108. doi: 10.1016/j.bpj.2017.07.021. Biophys J. 2017. PMID: 28877492 Free PMC article.
-
Anatomical and functional gonadotrope networks in the teleost pituitary.Sci Rep. 2016 Mar 31;6:23777. doi: 10.1038/srep23777. Sci Rep. 2016. PMID: 27029812 Free PMC article.
-
Direct association of connexin36 with zonula occludens-2 and zonula occludens-3.Neurochem Int. 2009 May-Jun;54(5-6):393-402. doi: 10.1016/j.neuint.2009.01.003. Neurochem Int. 2009. PMID: 19418635 Free PMC article.
-
A high-throughput fluorimetric microarray with enhanced fluorescence and suppressed "coffee-ring" effects for the detection of calcium ions in blood.Sci Rep. 2016 Dec 5;6:38602. doi: 10.1038/srep38602. Sci Rep. 2016. PMID: 27917959 Free PMC article.
References
-
- Barbara J-G, Poncer J-C, McKinney RA, Takeda K. An adrenal slice preparation for the study of chromaffin cells and their cholinergic innervation. J Neurosci Methods. 1998;80:181–189. - PubMed
-
- Belluardo N, Mudò G, Trovato-Salinaro A, Le Gurun S, Charollais A, Serre-Beinier V, Amato G, Haefliger JA, Meda P, Condorelli DF. Expression of connexin36 in the adult and developing rat brain. Brain Res. 2000;865:121–138. - PubMed
-
- Bonnefont X, Fiekers J, Creff A, Mollard P. Rhythmic bursts of calcium transients in acute anterior pituitary slices. Endocrinology. 2000;141:868–875. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
